The pSmad1/4 Page
The tumor suppressor Smad4/DPC4 is regulated by phosphorylations that integrate FGF, Wnt, and TGF-ß
Hadrien Demagny, Tatsuya Araki and Edward M. De Robertis
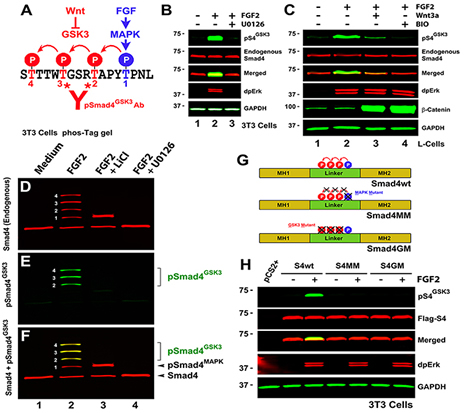 |
Figure 1. The Smad4 Linker Region is Phosphorylated by GSK3 |
|---|---|
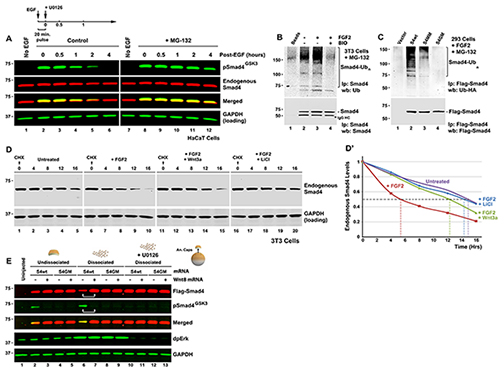 |
Figure 2. Wnt-regulated GSK3 Phosphorylations Control Smad4 Polyubiquitination and Degradation (A) Time-course of pSmad4GSK3 phosphorylation primed by a 20 min pulse of EGF in HaCaT cells showing that the proteasome inhibitor MG-132 preferentially prolongs the half-life of pSmad4GSK3. (B) Endogenous Smad4 polyubiquitination is increased by FGF and requires GSK3 activity (in the presence of the proteasome inhibitor MG132). Before immunoprecipitation with monoclonal Smad4 antibody, 0.2% SDS was added, samples heated at 95oC for 10 min to break protein-protein interactions, and diluted 10-fold with RIPA buffer to ensure that the polyubiquitinated bands detected were not ubiquitinated Smad4-interacting proteins (Zhu et al., 1999). For 5% input loading see Figure S2A. (C) Smad4 polyubiquitination requires intact MAPK and GSK3 sites; Flag-Smad4 or its phosphorylation-resistant mutants were co-transfected with HA-Ubiquitin into FGF-treated HEK293 cells and immunoprecipitated (Zhu et al., 1999) with anti-Flag antibodies. For 5% input loading see Figure S2B. (D) Wnt3a or LiCl treatment extended the half-life of endogenous Smad4 exclusively in FGF-treated 3T3 cells. Note that in the absence of FGF, Smad4 has a longer half-life. Similar results were obtained in two independent experiments. (D’) Quantification of western results shown in panel D. (E) Smad4 protein is stabilized by microinjection of xWnt8 mRNA in Xenopus dissociated animal cap cells. In dissociated cells dpErk is activated, causing increased pSmad4GSK3 and Flag-Smad4 degradation (lane 6). Both GSK3 phosphorylation and Flag-Smad4 degradation were blocked by co-injection of Wnt8 mRNA (lane 7). Smad4 degradation in microinjected embryos required intact GSK3 phosphorylation sites and was blocked by the Erk pathway inhibitor U0126 (40 μM). Cells were harvested at stage 10.5, early gastrula. |
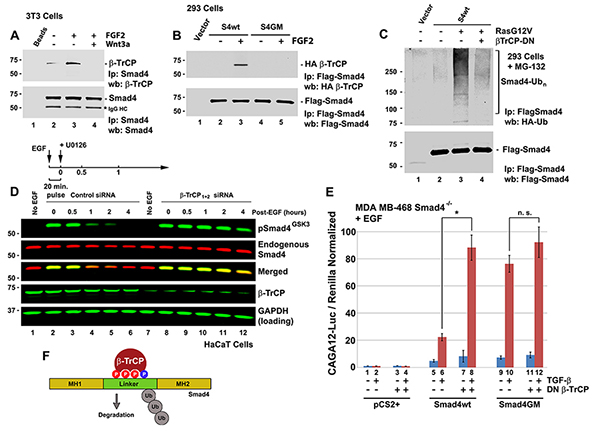 |
Figure 3. The Wnt-regulated Smad4 GSK3 phosphodegron is bound by the ubiquitin E3-ligase β-TrCP and targeted for degradation. |
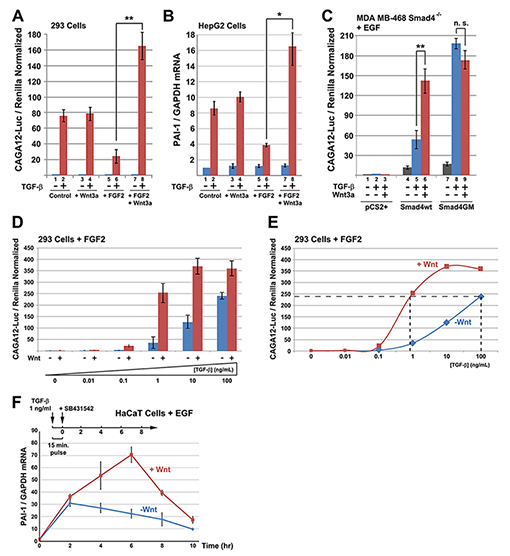 |
Figure 4. Wnt and TGF-β Signaling Cross-talk via Smad4 GSK3 Phosphorylations |
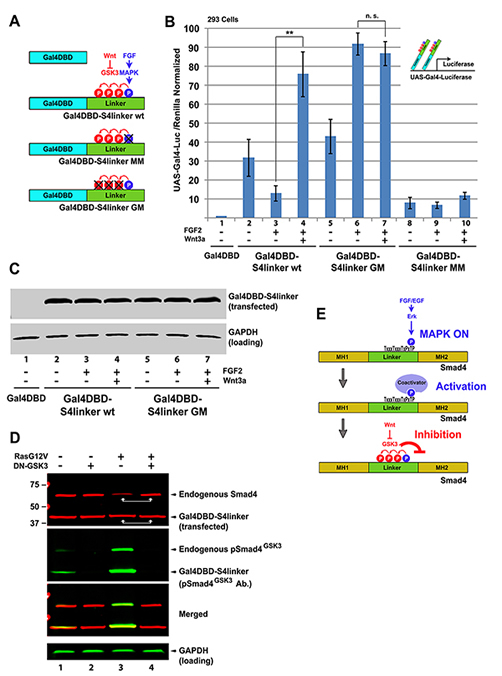 |
Figure 5. The Smad4 Linker Domain Contains a Growth Factor Regulated Transcriptional Activation Domain |
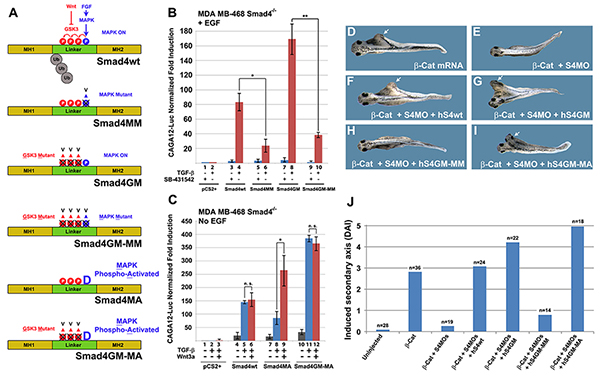 |
Figure 6. Phosphorylation of Threonine 277 is Required for Smad4 Peak Activity |
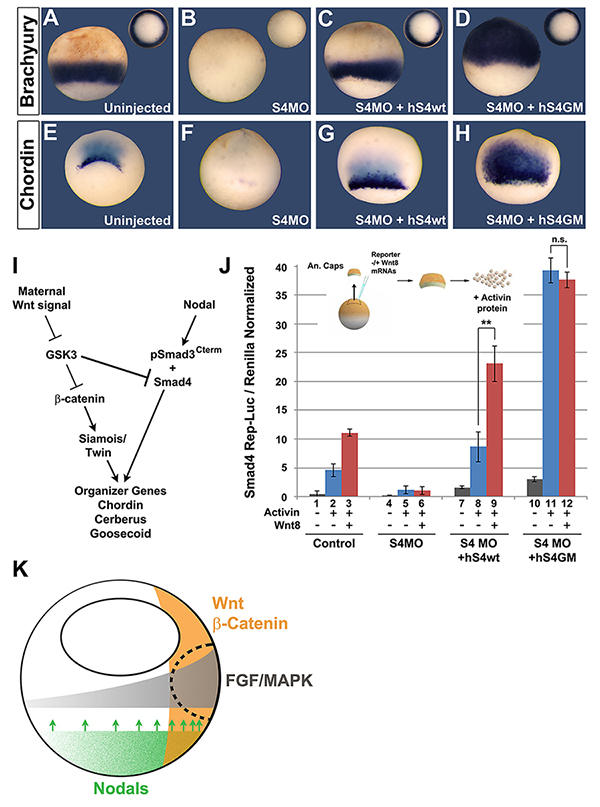 |
Figure 7. Smad4 Regulation by GSK3/Wnt is Involved in Germ-Layer Specification and organizer formation in Xenopus |
Integrating patterning signals: Wnt/GSK3 regulates the duration of the BMP/Smad1 signal
Luis C. Fuentealba, Edward Eivers, Atsushi Ikeda, Cecilia Hurtado, Hiroki Kuroda, Edgar M. Pera and E. M. De Robertis
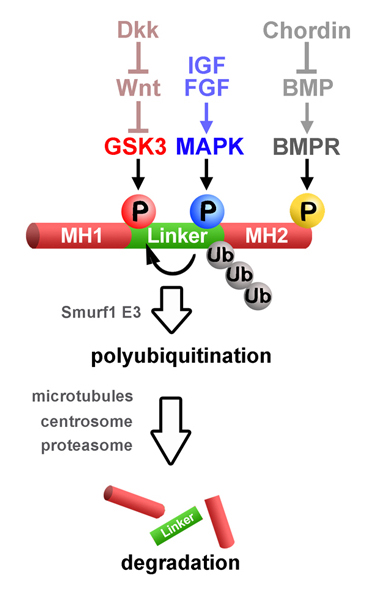
BMP receptors determine the intensity of BMP signals via Smad1 C-terminal phosphorylations. Here we show that a finely controlled cell biological pathway terminates this activity. The duration of the activated pSmad1(Cter) signal was regulated by sequential Smad1 linker region phosphorylations at conserved MAPK and GSK3 sites required for its polyubiquitinylation and transport to the centrosome. Proteasomal degradation of activated Smad1 and total polyubiquitinated proteins took place in the centrosome. Inhibitors of the Erk, p38, and JNK MAPKs, as well as GSK3 inhibitors, prolonged the duration of a pulse of BMP7. Wnt signaling decreased pSmad1(GSK3) antigen levels and redistributed it from the centrosome to cytoplasmic LRP6 signalosomes. In Xenopus embryos, it was found that Wnts induce epidermis and that this required an active BMP-Smad pathway. Epistatic experiments suggested that the dorsoventral (BMP) and anteroposterior (Wnt/GSK3) patterning gradients are integrated at the level of Smad1 phosphorylations during embryonic pattern formation.
 |
Wnt, IGF and FGF regulate Smad1 phosphorylation |
Integration of IGF, FGF and anti-BMP signals via Smad1 phosphorylation in neural induction
Edgar M. Pera, Atsushi Ikeda, Edward Eivers and E. M. De Robertis
Genes Dev. 17, 3023-3028 (2003)
How do very diverse signaling pathways induce neural differentiation in Xenopus? Anti-BMP (Chordin), FGF8, and IGF2 signals are integrated in the embryo via the regulation of Smad1 phosphorylation. Neural induction results from the combined inhibition of BMP receptor serine/threonine kinases and activation of receptor tyrosine kinases that signal through MAPK and phosphorylate Smad1 in the linker region, further inhibiting Smad1 transcriptional activity. This hard-wired molecular mechanism at the level of the Smad1 transcription factor may help explain the opposing activities of IGF, FGF, and BMP signals not only in neural induction, but also in other aspects of vertebrate development.
Depletion of Bmp2, Bmp4, Bmp7 and Spemann organizer signals induces massive brain formation in Xenopus embros
Bruno Reversade, Hiroki Kuroda, Hojoon Lee, Ashley Mays and E. M. De Robertis
Development 132, 3381-3392 (2005)
To address the patterning function of the Bmp2, Bmp4 and Bmp7 growth factors, we designed antisense morpholino oligomers (MO) that block their activity in Xenopus laevis. Bmp4 knockdown was sufficient to rescue the ventralizing effects caused by loss of Chordin activity. Double Bmp4 and Bmp7 knockdown inhibited tail development. Triple Bmp2/Bmp4/Bmp7 depletion further compromised trunk development but did not eliminate dorsoventral patterning. Unexpectedly, we found that blocking Spemann organizer formation by UV treatment or beta-Catenin depletion caused BMP inhibition to have much more potent effects, abolishing all ventral development and resulting in embryos having radial central nervous system (CNS) structures. Surprisingly, dorsal signaling molecules such as Chordin, Noggin, Xnr6 and Cerberus were not re-expressed in these embryos. We conclude that BMP inhibition is sufficient for neural induction in vivo, and that in the absence of ventral BMPs, Spemann organizer signals are not required for brain formation.
Regulation of ADMP and BMP2/4 at opposite embryonic poles generates a self-regulating morphogen field
Bruno Reversade and E. M. De Robertis
Embryos have the ability to self-regulate and regenerate normal structures after being sectioned in half. How is such a morphogenetic field established? We discovered that quadruple knockdown of ADMP and BMP2/4/7 in Xenopus embryos eliminates self-regulation, causing ubiquitous neural induction throughout the ectoderm. ADMP transcription in the Spemann organizer is activated at low BMP levels. When ventral BMP2/4/7 signals are depleted, Admp expression increases, allowing for self-regulation. ADMP has BMP-like activity and signals via the ALK-2 receptor. It is unable to signal dorsally because of inhibition by Chordin. The ventral BMP antagonists Sizzled and Bambi further refine the pattern. By transplanting dorsal or ventral wild-type grafts into ADMP/BMP2/4/7-depleted hosts, we demonstrate that both poles serve as signaling centers that can induce histotypic differentiation over considerable distances. We conclude that dorsal and ventral BMP signals and their extracellular antagonists expressed under opposing transcriptional regulation provide a molecular mechanism for embryonic self-regulation.
Default neural induction: neuralization of dissociated Xenopus cells is mediated by Ras/MAPK activation
Hiroki Kuroda, Luis Fuentealba, Atsushi Ikeda, Bruno Reversade and E. M. De Robertis
Genes Dev. 19, 1022-1027 (2005)
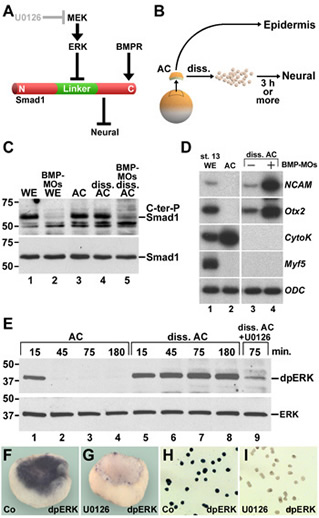
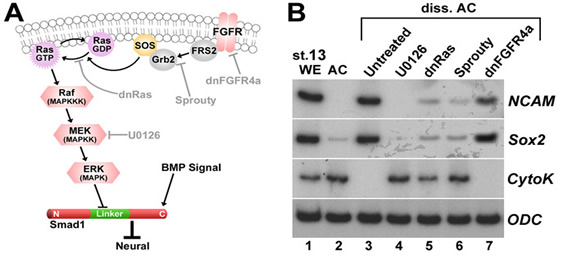
Xenopus embryonic ectodermal cells dissociated for three or more hours differentiate into neural tissue instead of adopting their normal epidermal fate. This default type of neural induction occurs in the absence of Spemann organizer signals and is thought to be caused by the dilution of endogenous BMPs into the culture medium. Unexpectedly, we observed that BMP ligands continue to signal in dissociated cells. Instead, cell dissociation induces a sustained activation of the Ras/MAPK pathway, which causes the phosphorylation of Smad1 at sites that inhibit the activity of this transcription factor. It is this activation of Ras/MAPK that is required for neuralization in dissociated ectoderm.
 |
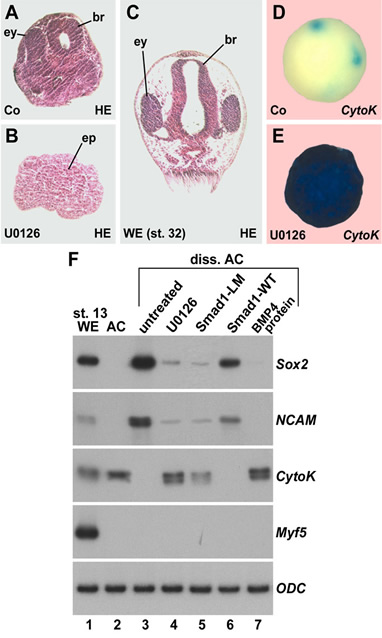 |
Figure 1. Cell dissociation does not cause BMP depletion by dilution, but rather triggers sustained ERK/MAPK activation in Xenopus ectodermal cells |
Figure 2. Histological sections, whole-mount in situ hybridizations, and RT-PCR analyses showing that inhibition of ERK blocks neural induction in dissociated animal cap cells. |
 |
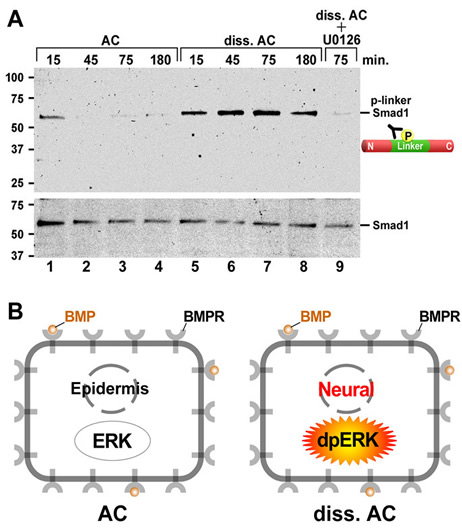 |
Figure 3. Dissociation activates much of the Ras/MAPK pathway. (A) Diagram of ERK pathway components. Dimerized FGF receptors (FGFR) become phosphorylated and recruit the Grb2-SOS complex via FGFR substrate protein 2 (FRS2). | Figure 4. Cell dissociation causes linker-specific phosphorylation of Smad1 in Xenopus ectoderm. |
Spemann's organizer and self-regulation in amphibian embryos
E. M. De Robertis
Nat. Rev. Mol. Cell Biol. 7, 296-302 (2006)
In 1924, Spemann and Mangold demonstrated the induction of Siamese twins in transplantation experiments with salamander eggs. Recent work in amphibian embryos has followed their lead and uncovered that cells in signalling centres that are located at the dorsal and ventral poles of the gastrula embryo communicate with each other through a network of secreted growth-factor antagonists, a protease that degrades them, a protease inhibitor and bone-morphogenic-protein signals.
 |
Embryonic self-regulation |
Neural induction in the absence of organizer in salamanders is mediated by MAPK
Cecilia Hurtado and E. M. De Robertis
Dev. Biol. 307, 282-289 (2007)
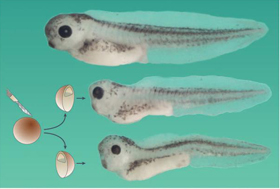
Research on the mechanisms of embryonic induction had a great setback in the 1940s when Barth discovered and Holtfreter confirmed that ectoderm of Ambystoma maculatum salamander embryos could form brain tissue when cultured in a simple saline solution. We have revisited this classical experiment and found that when cultured animal cap ectoderm attaches to a glass substratum, it can self-organize to form complex organs such as brain vesicles, eyes, lens and olfactory placodes. Only anterior neural organs were generated. Under these culture conditions ERK became diphosphorylated, indicating a sustained activation of the Ras/MAPK pathway. Using sand particles as an example of a heterologous neural inducer similar results were obtained. Addition of U0126, a specific antagonist of MEK, the enzyme that phosphorylates ERK/MAPK, inhibited neural differentiation. The closely related control compound U0124 had no effect. We conclude that neural induction in the absence of organizer in A. maculatum requires Ras/MAPK-activation. These findings provide a molecular explanation for the activity of heterologous neural inducers that dominated thinking in amphibian experimental embryology for many decades.
 |
Organs and histotypes that differentiate in Ambystoma maculatum ectoderm cultured in Holfreter's saline solution. |
Asymmetric mitosis: Unequal segregation of proteins destined for degradation
Luis C. Fuentealba, Edward Eivers, Douglas Geissert, Vincent Taelman and E. M. De Robertis
Proc. Natl. Acad. Sci. USA 105, 7732-7737 (2008)
Mitotic cell division ensures that two daughter somatic cells inherit identical genetic material. Previous work has shown that signaling by the Smad1 transcription factor is terminated by polyubiquitinylation and proteasomal degradation after essential phosphorylations by MAPK and glycogen synthase kinase 3 (GSK3). Here, we show that, unexpectedly, proteins specifically targeted for proteasomal degradation are inherited preferentially by one mitotic daughter during somatic cell division. Experiments with dividing human embryonic stem cells and other mammalian cultured cell lines demonstrated that in many supposedly equal mitoses the segregation of proteins destined for degradation (Smad1 phosphorylated by MAPK and GSK3, phospho-beta-catenin, and total polyubiquitinylated proteins) was asymmetric. Transport of pSmad1 targeted for degradation to the centrosome required functional microtubules. In vivo, an antibody specific for Mad phosphorylated by MAPK showed that this antigen was associated preferentially with one of the two centrosomes in Drosophila embryos at cellular blastoderm stage. We propose that this remarkable cellular property may be explained by the asymmetric inheritance of peripheral centrosomal proteins when centrioles separate and migrate to opposite poles of the cell, so that one mitotic daughter remains pristine. We conclude that many mitotic divisions are unequal, unlike what was previously thought.
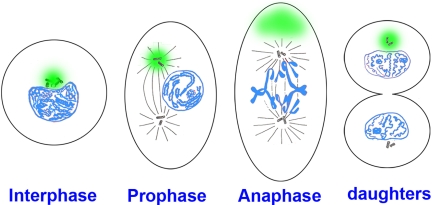 |
Cellular model in which the pericentrosomal material (indicated in green) is inherited asymmetrically at mitosis. At interphase, the centrosome and centrioles are surrounded by pericentrosomal material in which proteins destined for degradation concentrate (in this cell the centrosome is located in a nuclear bay cytoplasmic region). During mitosis the centrioles and associated centrosomal MTOC proteins (such as γ-tubulin and pericentrin) divide equally, whereas the pericentrosomal material remains in one pole.We propose that this asymmetry may provide dividing cell populations with a simple physiological mechanism to cleanse themselves of proteins destined for degradation at each cell division. |
Integrating positional information at the level of Smad1/5/8
Edward Eivers, Luis C. Fuentealba and E. M. De Robertis
Curr. Opin. Genet. Dev. 18, 304-310 (2008)
The intensity of the BMP signal is determined by cell surface receptors that phosphorylate Smad1/5/8 at the C-terminus. In addition to this BMP-activated phosphorylation, recent studies have shown that sequential phosphorylations by MAPK and GSK3 kinases can negatively regulate the activity of the pSmad1(Cter) signal. These phosphorylations in the linker region cause Smad1 to be transported to the centrosomal region, polyubiquitinylated and degraded by the proteasomal machinery. In Xenopus embryos, Wnt signals, which regulate GSK3, induce ectoderm to adopt an epidermal fate, and this Wnt effect requires an active BMP-Smad1/5/8 signaling pathway. These findings have profound implications for understanding how dorsal-ventral and anterior-posterior patterning are seamlessly integrated in the early embryonic morphogenetic field.
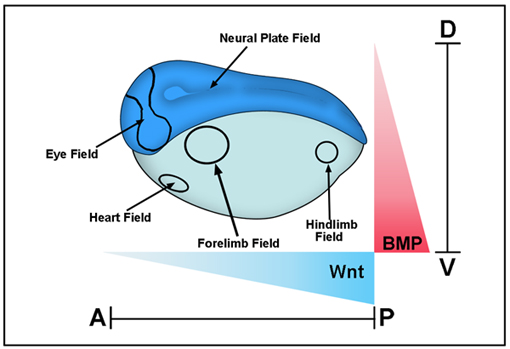 |
Xenopus gastrula stage embryo showing some of the main morphogenetic fields that form along the D–V (BMP) and A–P (Wnt) axes. These fields are considered self-regulating (as is the early blastula embryo as well) because they can be cut in half and regenerate the whole pattern after transplantation. BMP signals are high in the ventral side and decrease dorsally, while Wnt signals are strong in the posterior and weaken anteriorly. Cells at different positions within these two Cartesian axes read these morphogen gradients and determine the embryonic body plan that specifies the place at which the various organs will be subsequently formed. |
Mad is required for wingless signaling in wing development and segment patterning in Drosophila
Edward Eivers, Luis C. Fuentealba, Veronika Sander, James C. Clemens, Lori Hartnett and E. M. De Robertis
A key question in developmental biology is how growth factor signals are integrated to generate pattern. In this study we investigated the integration of the Drosophila BMP and Wingless/GSK3 signaling pathways via phosphorylations of the transcription factor Mad. Wingless was found to regulate the phosphorylation of Mad by GSK3 in vivo. In epistatic experiments, the effects of Wingless on wing disc molecular markers (senseless, distalless and vestigial) were suppressed by depletion of Mad with RNAi. Wingless overexpression phenotypes, such as formation of ectopic wing margins, were induced by Mad GSK3 phosphorylation-resistant mutant protein. Unexpectedly, we found that Mad phosphorylation by GSK3 and MAPK occurred in segmental patterns. Mad depletion or overexpression produced Wingless-like embryonic segmentation phenotypes. In Xenopus embryos, segmental border formation was disrupted by Smad8 depletion. The results show that Mad is required for Wingless signaling and for the integration of gradients of positional information.
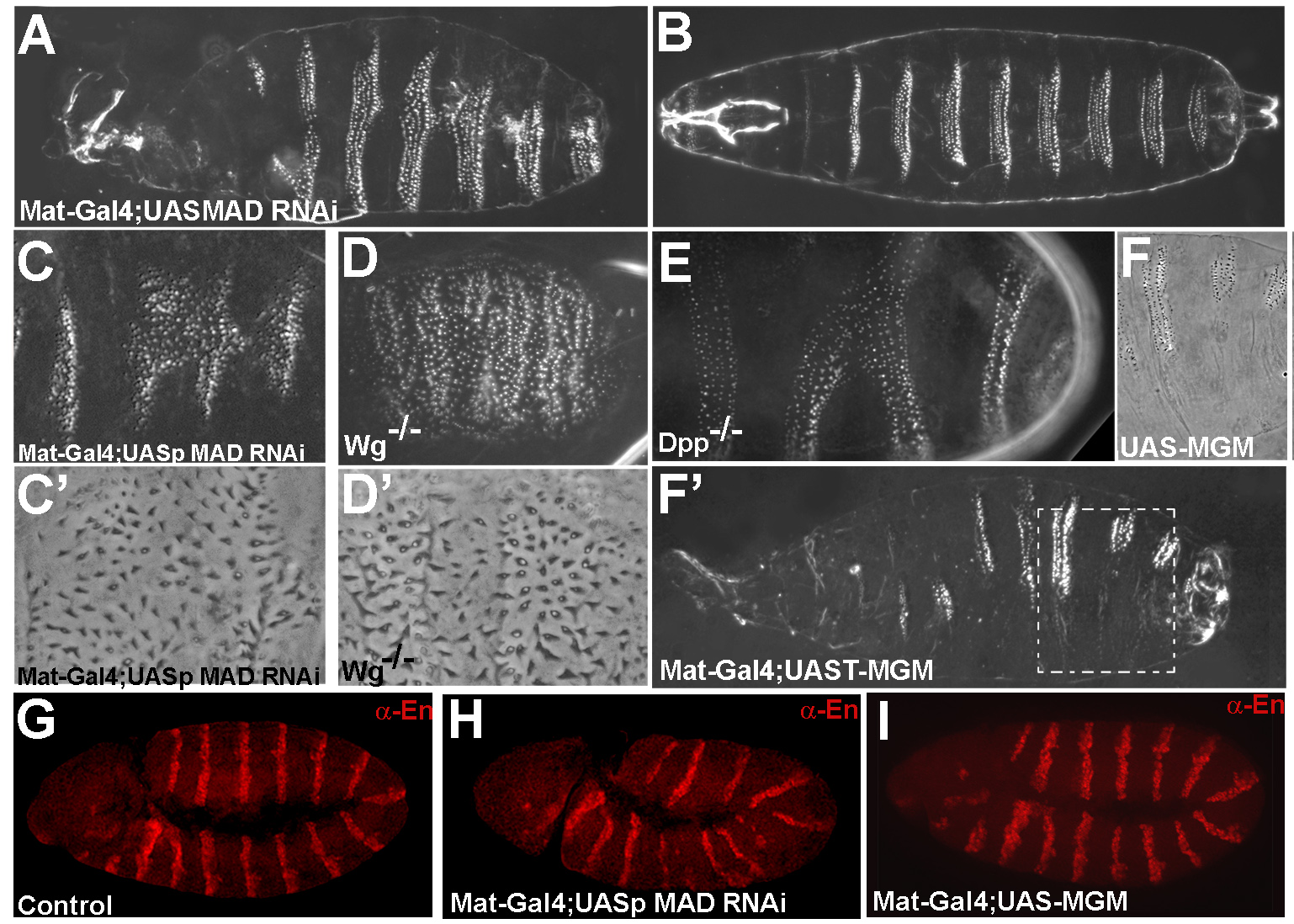 |
Mad is required for segmentation in Drosophila |
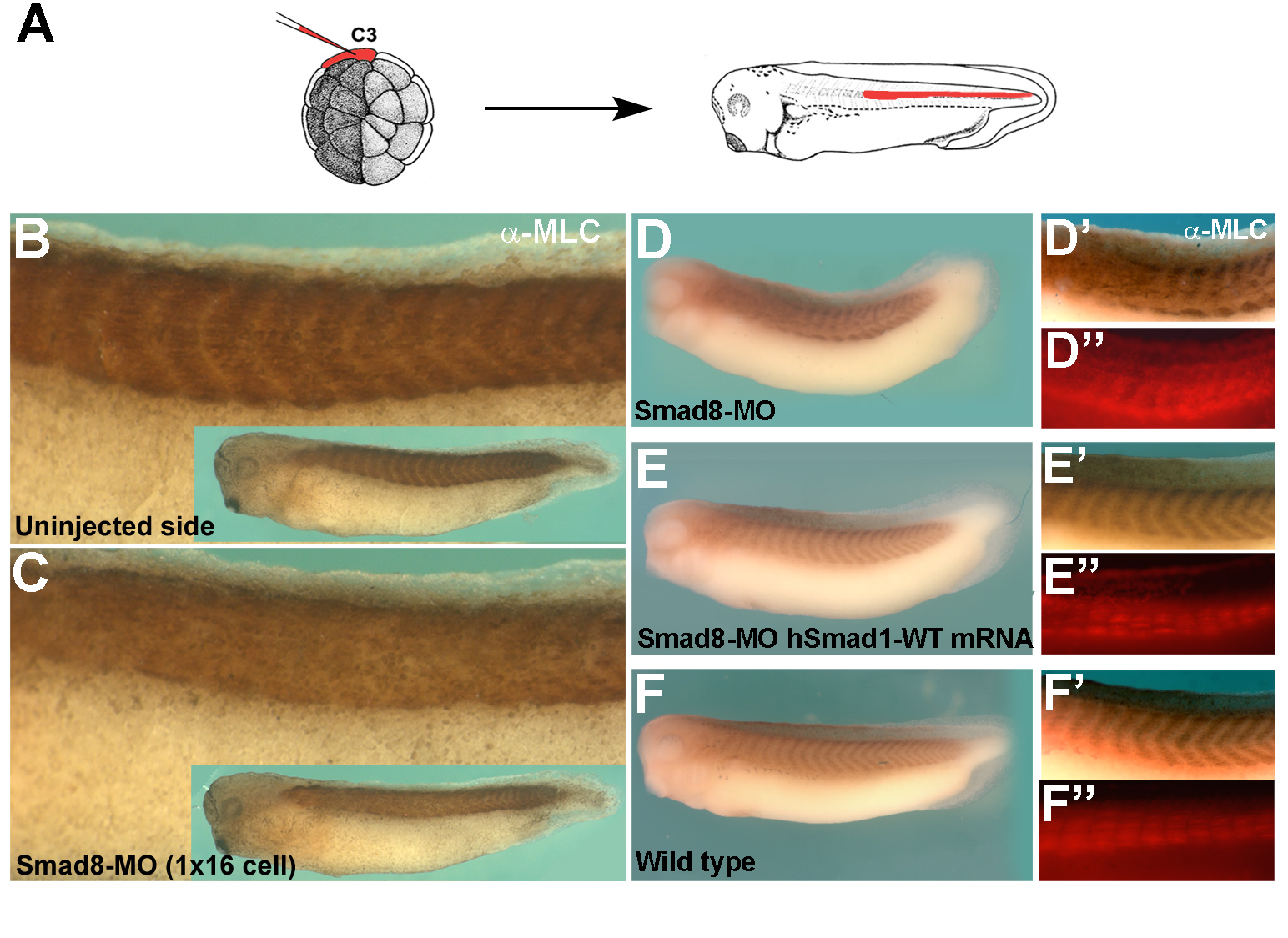 |
Smad5/8 is required for segment border formation in Xenopus embryos |
Updated: March 2019
