Figure 6. Sizzled, Xlr, and Chordin interact at the physiological 10-8M range
Hojoon X. Lee, Andrea L. Ambrosio, Bruno Reversade and E. M. De Robertis
Here we report an unexpected role for the secreted Frizzled-related protein (sFRP) Sizzled/Ogon as an inhibitor of the extracellular proteolytic reaction that controls BMP signaling during Xenopus gastrulation. Microinjection experiments suggest that the Frizzled domain of Sizzled regulates the activity of Xolloid-related (Xlr), a metalloproteinase that degrades Chordin, through the following molecular pathway: Szl -| Xlr -| Chd -| BMP --> P-Smad1 --> Szl. In biochemical assays, the Xlr proteinase has similar affinities for its endogenous substrate Chordin and for its competitive inhibitor Sizzled, which is resistant to enzyme digestion. Extracellular levels of Sizzled and Chordin in the gastrula embryo and enzyme reaction constants were all in the 10(-8) M range, consistent with a physiological role in the regulation of dorsal-ventral patterning. Sizzled is also a natural inhibitor of BMP1, a Tolloid metalloproteinase of medical interest. Furthermore, mouse sFRP2 inhibited Xlr, suggesting a wider role for this molecular mechanism.
 |
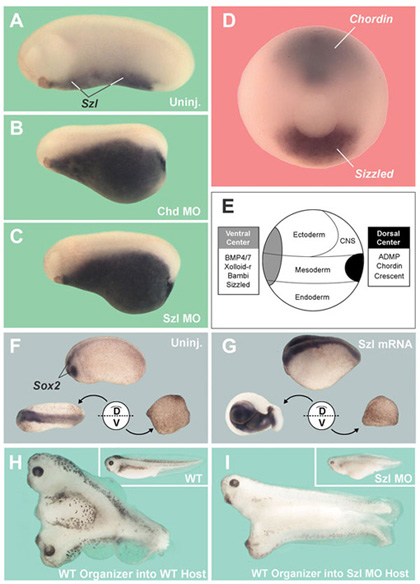
Figure 1. Sizzled is expressed ventrally yet requires a dorsal component |
 |
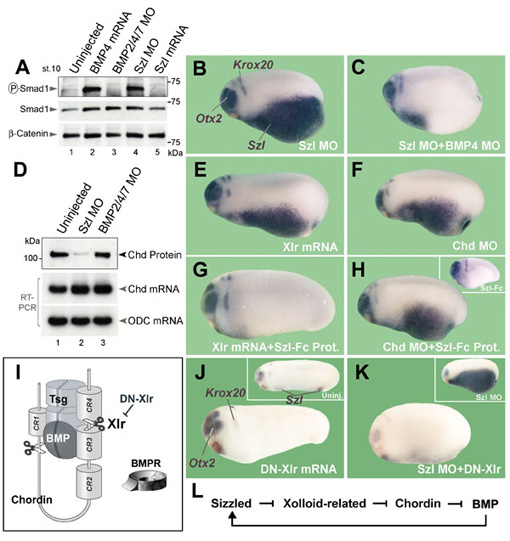
Figure 2. Sizzled is a feedback inhibitor of BMP signaling that requires Xlr activity |
 |
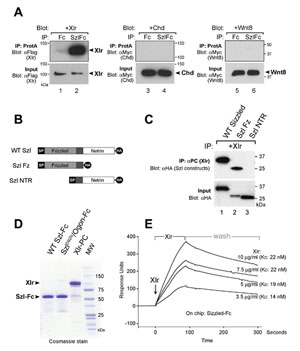
Figure 3. Sizzled binds to Xolloid-related |
 |
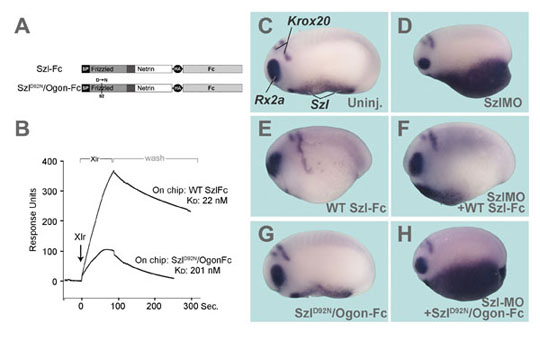
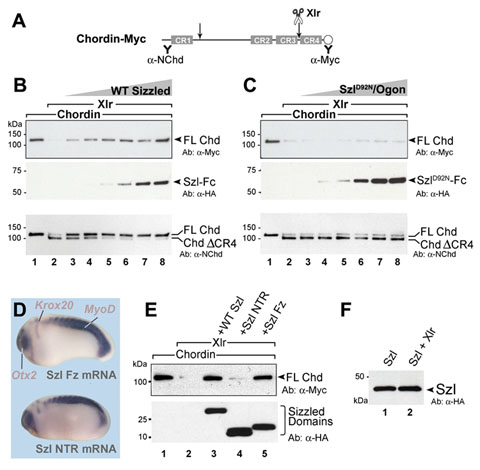
Figure 4. The Szl-D92N point mutation mimicking zebrafish ogon inhibits Xlr binding and biological activity |
 |
Figure 5. Sizzled, but not Szl-D92N mutant, inhibits the proteolytic activity of Xlr on its natural substrate Chordin |
 |
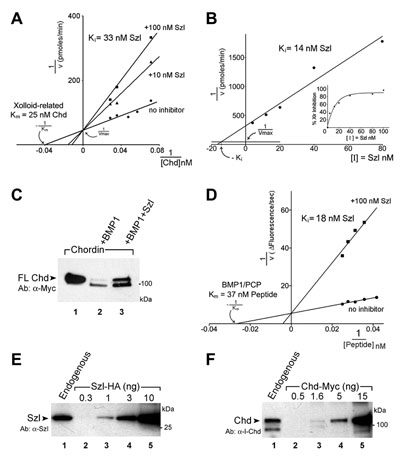
Figure 6. Sizzled, Xlr, and Chordin interact at the physiological 10-8M range |
 |
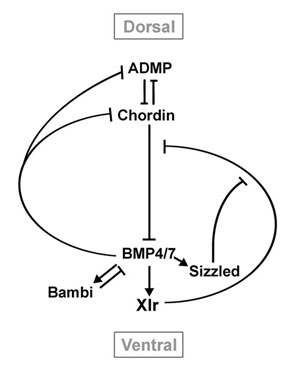
Figure 7. Model of the regulation of DV patterning in Xenopus embryos by a network of extracellulr proteins |