The Chordin
Page
The Chordin Morphogenetic Pathway
Edward M. De Robertis and Yuki Moriyama
Curr Top Dev Biol 116, 231-245 (2016)
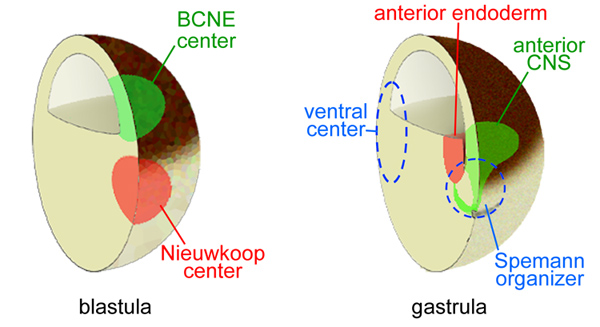
The ancestral Chordin/bone morphogenetic protein (BMP) signaling pathway that establishes dorsal-ventral (D-V) patterning in animal development is one of the best understood morphogenetic gradients, and is established by multiple proteins that interact with each other in the extracellular space-including several BMPs, Chordin, Tolloid, Ont-1, Crossveinless-2, and Sizzled. The D-V gradient is adjusted redundantly by regulating the synthesis of its components, by direct protein-protein interactions between morphogens, and by long-range diffusion. The entire embryo participates in maintaining the D-V BMP gradient, so that for each action in the dorsal side there is a reaction in the ventral side. A gradient of Chordin is formed in the extracellular matrix that separates ectoderm from endomesoderm, called Brachet's cleft in Xenopus. The Chordin/BMP pathway is self-organizing and able to scale pattern in the dorsal half of bisected embryos or in Spemann dorsal lip transplantation experiments.
Chordin forms a self-organizing morphogen gradient in the extracellular space between ectoderm and mesoderm in the Xenopus embryo
Jean-Louis Plouhinec, Lise Zakin, Yuki Moriyama and E. M. De Robertis
PNAS 110, 29372-20379 (2013)
The vertebrate body plan follows a stereotypical dorsal-ventral (D-V) tissue differentiation controlled by Bone Morphogenetic proteins (BMP) and secreted BMP antagonists such as Chordin. The three germ layers – ectoderm, mesoderm and endoderm – are affected coordinately by the Chordin/BMP morphogen system. However, extracellular morphogen gradients of endogenous proteins have not been directly visualized in vertebrate embryos to date. In this study, we improved immunolocalization methods in Xenopus embryos and analyzed the distribution of endogenous Chordin using a specific antibody. Chordin protein secreted by the dorsal Spemann organizer was found to diffuse along a narrow region that separates the ectoderm from anterior endoderm and mesoderm. This Fibronectin-rich extracellular matrix (ECM) is called Brachet’s cleft in the Xenopus gastrula and is present in all vertebrate embryos. Chordin protein formed a smooth gradient that encircled the embryo, reaching the ventral-most Brachet cleft. Depletion with morpholino oligos showed that this extracellular gradient was regulated by the Chordin protease Tolloid and its inhibitor Sizzled. The Chordin gradient, as well as the BMP signaling gradient, was self-regulating and, importantly, was able to rescale in dorsal half-embryos. Transplantation of Spemann organizer tissue showed that Chordin diffused over long distances along this signaling highway between ectoderm and mesoderm. Chordin protein must reach very high concentrations in this narrow region. We suggest that as ectoderm and mesoderm undergo morphogenetic movements during gastrulation, cells in both germ layers read their positional information coordinately from a single morphogen gradient located in Brachet’s cleft.
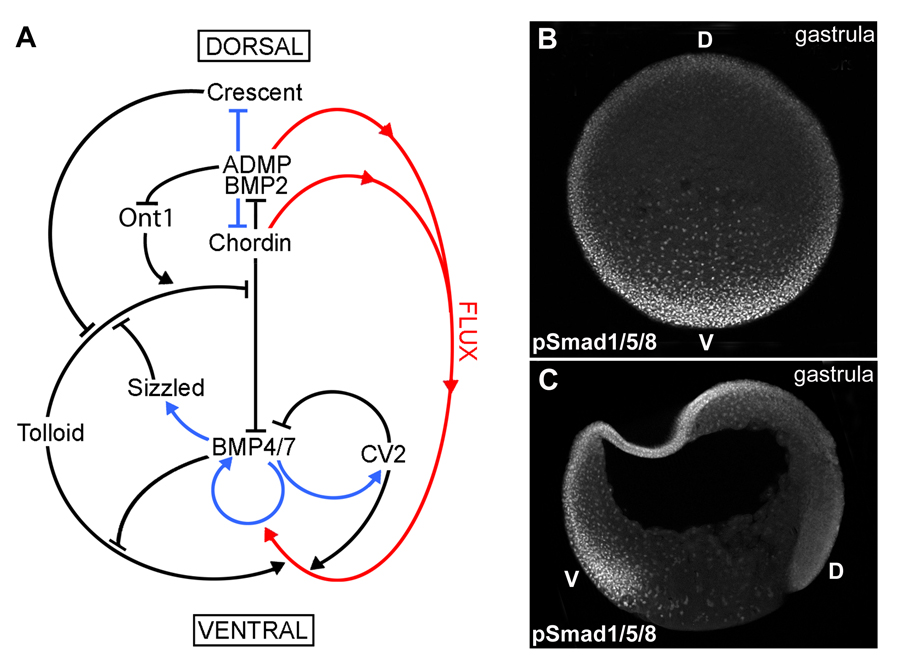 |
Fig. 1. A gradient of BMP activity patterns the D-V axis of the Xenopus gastrula.
(A) BMP activity along the D-V axis results from a series of direct protein-protein interactions between Chordin and other partners (black arrows), transcriptional regulation (blue arrows), and protein flux (red arrows). The entire embryo participates in forming the BMP gradient, which results from the dueling activities of the dorsal and ventral signaling centers.
(B) Transverse optical section at gastrula (stage 11) showing a ventral (V) to dorsal (D) gradient of BMP activity using anti-pSmad1/5/8 antibody as readout.
(C) Gastrula (stage 11) embryo sectioned sagittaly, showing higher BMP activity in the ventral animal cap and marginal zone nuclei as assessed by pSmad1/5/8 immunostaining. |
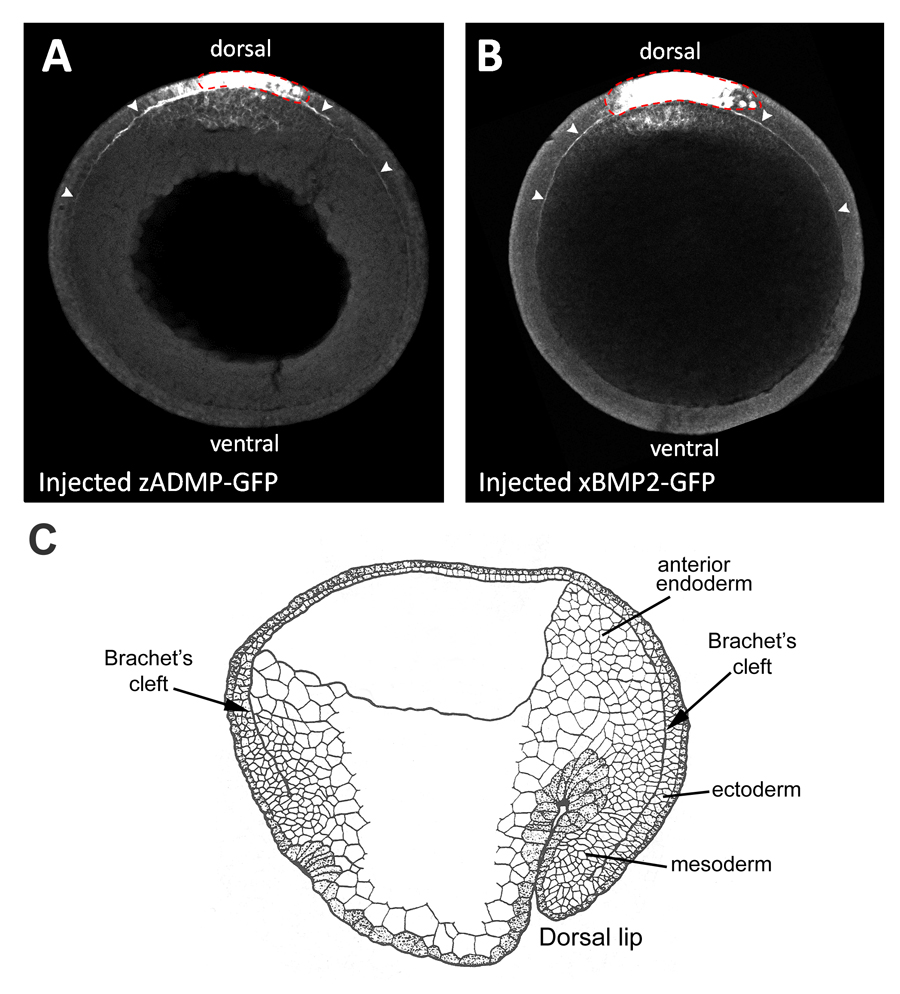 |
Fig. 2. Overexpressed BMP-GFP fusion proteins diffuse in Brachet’s cleft.
(A, B) Dorsally expressed zADMP-GFP and xBMP2-GFP fusion proteins diffuse within the narrow confines of Brachet’s cleft (marked by arrowheads) away from the point of mRNA injection in ectodermal cells (indicated by a red dotted line) (n=7 and n=6, respectively). GFP fusion proteins were detected by GFP immunostainings of transverse optical sections of stage 12 embryos through the animal-vegetal axis.
(C) Diagram of stage 12 sagittal section of a Xenopus embryo (after P. Nieuwkoop). Brachet’s cleft is the narrow cavity that separates the mesodermal and anterior endodermal layers from the ectoderm, and encircles the entire D-V axis. |
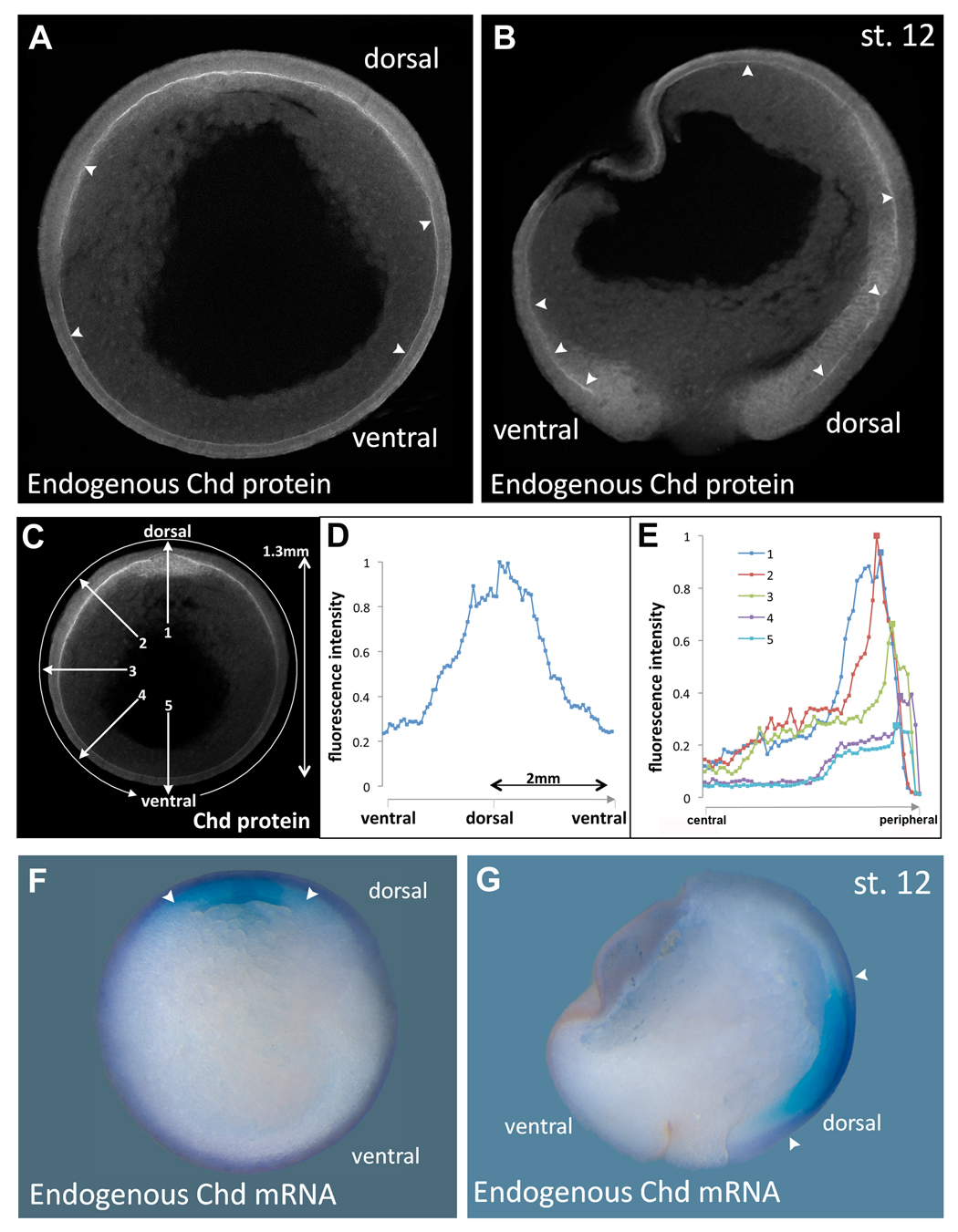 |
Fig. 3. Endogenous Chordin protein diffusion within Brachet’s cleft compared to the expression of chordin mRNA.
(A, B) Immunostainings of Chordin protein in late gastrula (stage 12) embryos in transverse (n=16) or sagittal (n=15) sections, respectively, using an affinity-purified Chordin antibody. Chordin protein is detected throughout the entire length of Brachet’s cleft (arrowheads) forming a gradient from dorsal towards ventral.
(C) Imaging of the Chordin gradient following the entire circumference in a clockwise manner (circular arrow) or in a radial manner (numbered arrows).
(D) Measurement of the Chordin gradient of the embryo seen in the previous panel. Note that the gradient forms over a very long distance of almost two millimeters; similar profiles were obtained in the four embryos analyzed.
(E) Intensity of fluorescence plotted along 5 radial lines from central to peripheral. Line 1 is dorsal and shows Chordin protein peaks in organizer mesoderm and in Brachet’s cleft. In the other lines a peak is seen in Brachet’s cleft, even in the ventral-most region.
(F, G) In situ hybridization of stage 12 embryo in transverse or sagittal section showing that chordin mRNA is transcribed only in the dorsal side (arrowheads); compare to the panels showing Chordin protein localization at the same stage. |
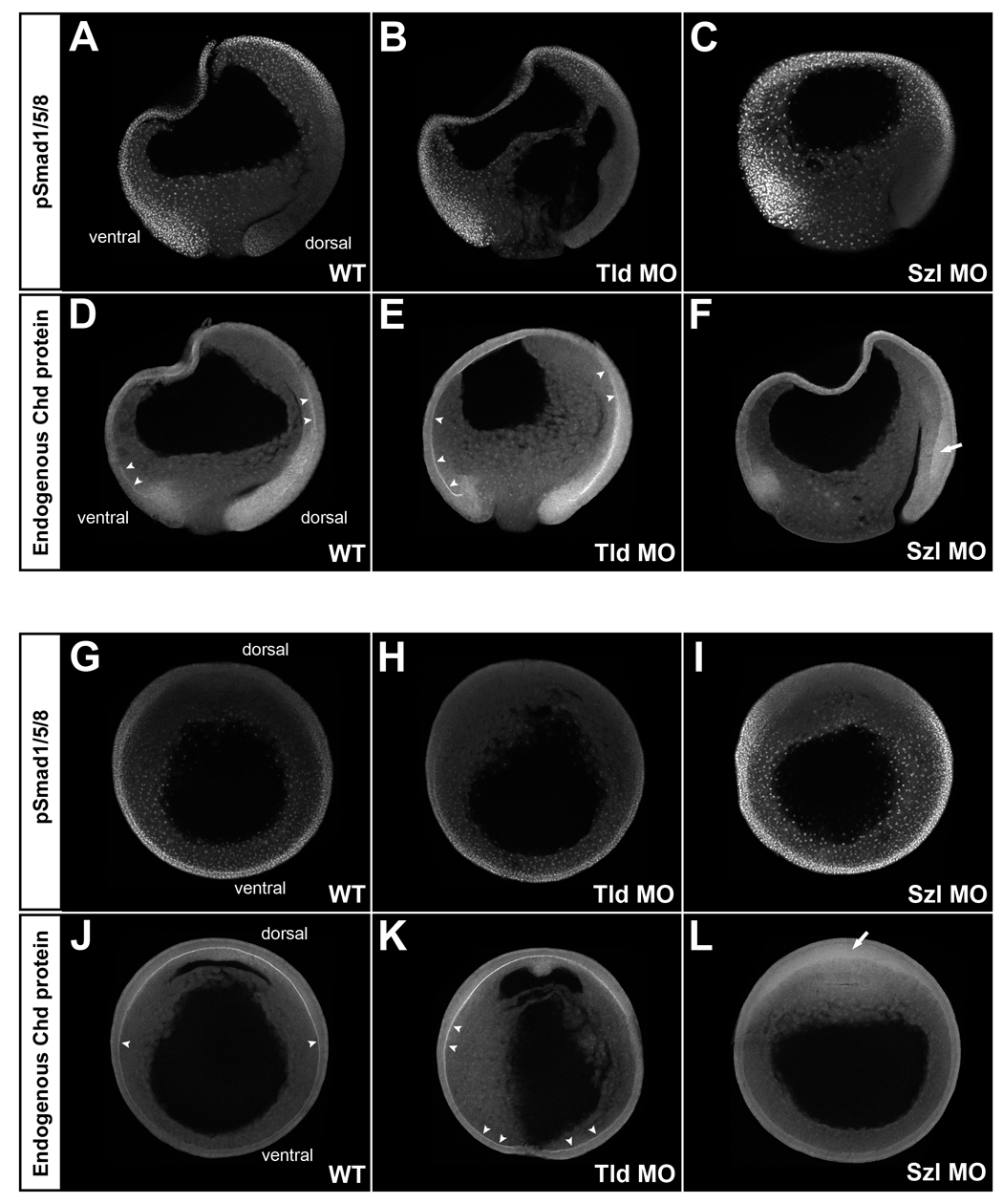 |
Fig. 4. Analysis of BMP signaling and Chordin protein localization in embryos depleted of Tolloid (Tld MO) or Sizzled (Szl MO). All embryos were siblings allowed to develop for the same period of time; all images were processed identically.
(A-F) pSmad1/5/8 (top panels) and Chordin (bottom panels) immunostainings of sagittal optical sections. The gradient of BMP activity was complementary to that of Chordin localization in WT embryos (left panels, n=6 and n=4, respectively). When translation of the Chordin-degrading enzyme Tolloid was inhibited in Tld MOs injected embryos (middle panels, n=5 and n=3, respectively), BMP activity was decreased on the dorsal side and increased accumulation of Chordin was observed in Brachet’s cleft (arrowheads). Conversely, when Sizzled was depleted (Szl MO), BMP activity (nuclear pSmad1/5/8) was greatly increased in the embryo, and Chordin failed to accumulate in the Brachet’s cleft (right panels, n=3). Diffuse accumulation of Chordin in dorsal ectoderm is indicated by the arrow.
(G-L) pSmad1/5/8 (top panels) and Chordin (bottom panels) immunostainings of embryos sectioned transversely through the animal-vegetal axis at late gastrula (stage 12). pSmad1/5/8 staining was decreased in Tld MO embryos (top middle panel, n=6) and increased in Szl MO embryos (top right panel, n=4) compared to WT (top left panel n=6). Tld MO increased (bottom middle panel n=7) and Szl MO decreased (bottom right panel, n=5) Chordin staining in Brachet’s cleft compared to WT (bottom left panel, n=7). |
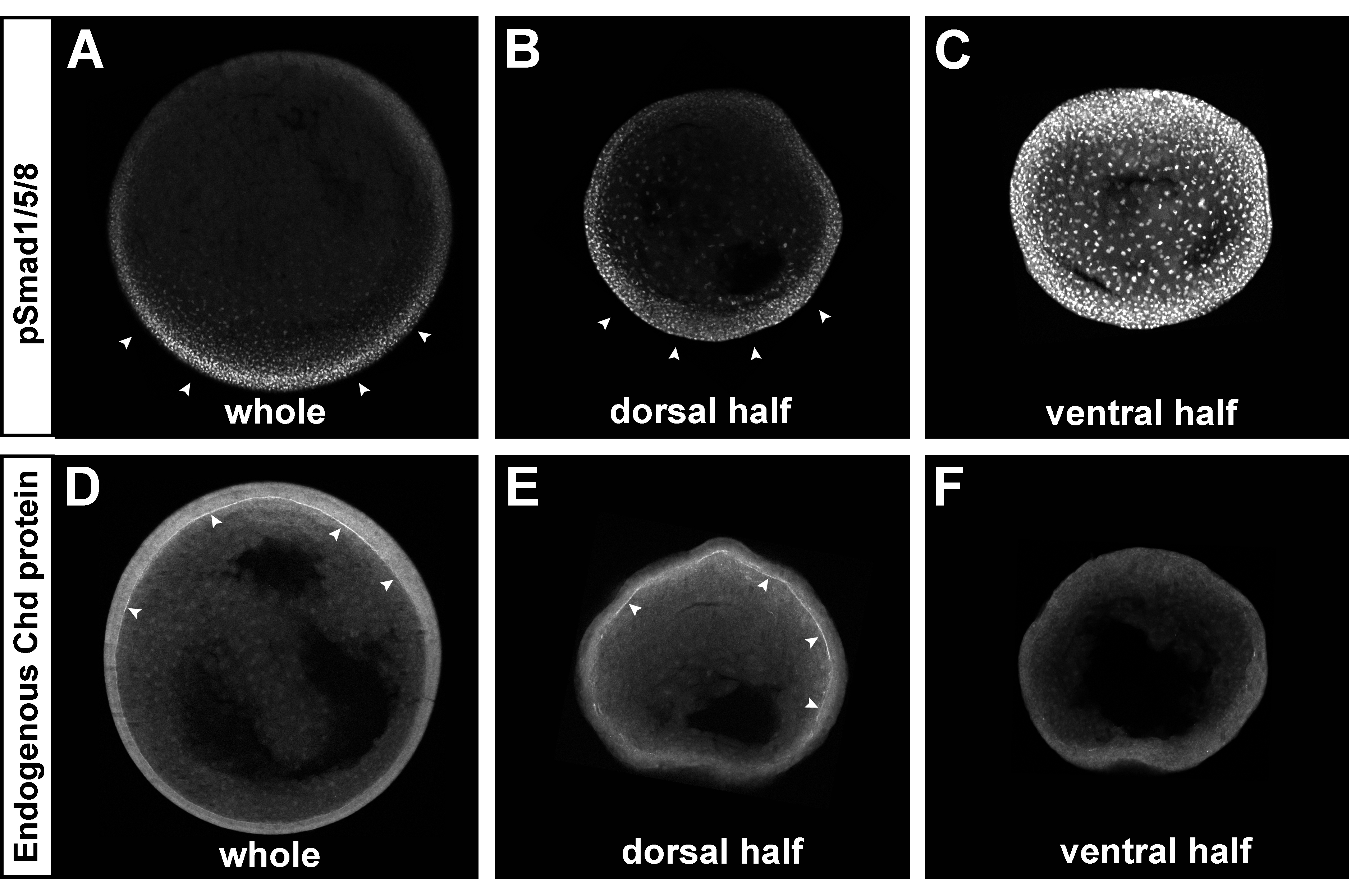 |
Fig. 5. The Chordin and pSmad1/5/8 gradients self-regulate in dorsal half-embryos. The ventral side of albino embryos was marked at the 8-cell stage using the new method described in Fig. S4. Embryos were bisected at stage 9, cultured until stage 12, fixed, and sectioned transversely. All embryos were siblings and images were processed identically.
(A-C) pSmad1/5/8 immunostaining of whole embryo (left, n=3), dorsal (middle, n=6) or ventral (right, n=3 out of 4 showing the phenotype) half-embryos. Note that the BMP gradient was reestablished in the dorsal half-embryo, while very strong uniform pSmad1/5/8 was present in the ventral half-embryo.
(D-F) Chordin immunostaining of whole embryo (left, n=3), dorsal (middle, n=10) or ventral (right, n=7) half-embryo. The Chordin gradient was regenerated in the Brachet’s cleft of dorsal half-embryos (in 8 out of 10), while no Chordin expression was detected in the ECM of the ventral half-embryo (in 6 out of 7). Similar results were obtained in two independent experiments. |
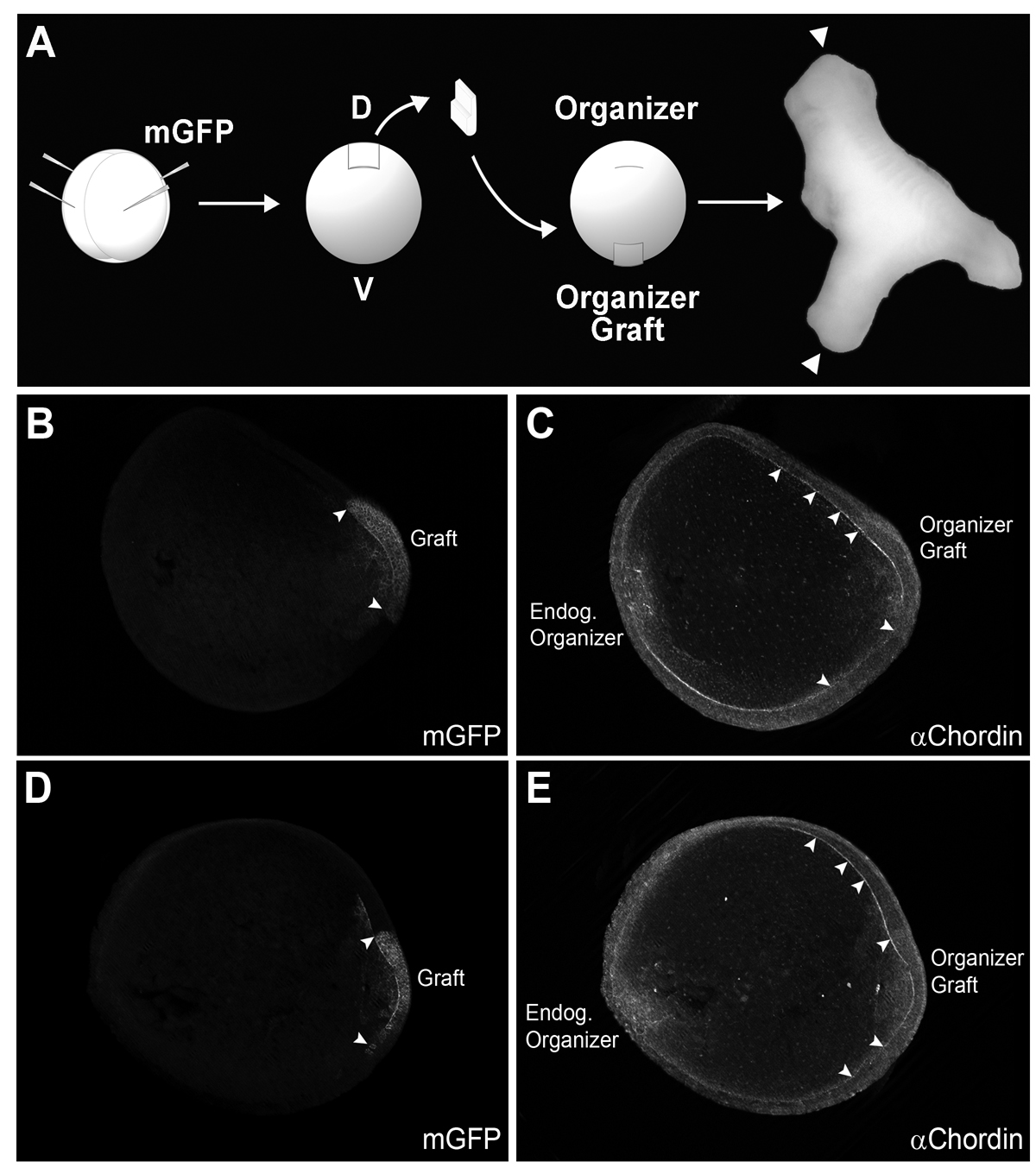 |
Fig. 6. Chordin protein diffuses long-range in Brachet’s cleft of embryos transplanted with Spemann organizer tissue.
(A) Diagram of experimental procedure. mGFP was injected in the donor embryo (4 injections at the 4-cell stage) to trace the lineage of grafted tissue. Secondary axes were complete (with heads, indicated with arrowheads in this photograph) in 75% of the cases (n=6/8).
(B-E) Two embryos transplanted at stage 10 and fixed at stage 12 stained with GFP and Chordin antibodies are shown in optical transverse sections. The left panel shows localization of the grafted organizer tagged with mGFP; borders are indicated by arrowheads. In the right panels, a second gradient of Chordin was observed, with Chordin protein staining in Brachet’s cleft extending a considerable distance from the graft (arrowheads) (n=19). This indicates that Chordin protein diffuses from the graft through Brachet’s cleft. |
Complexity and Analogy in Science
Pontifical Academy of Sciences, Acta 22, Vatican City 2014
www.pas.va/content/dam/accademia/pdf/acta22/acta22-derobertis.pdf
Deciphering Complexity in Biology:
Induction of Embryonic Cell Differentiation
by Morphogen Gradients
Edward M. De Robertis
Introduction
One of the most complex and mysterious processes in nature is the development of a vertebrate animal from a single cell into an organism consisting of hundreds of differentiated cell types that are reproducibly arranged in specific spatial patterns. Understanding developmental mechanisms is essential because it is during development that the information contained in the DNA (the genotype) is interpreted into morphological phenotypes.
Historically, developmental biology has attempted to study the embryo as a complete system that develops seamlessly into a perfectly shaped organism. For example, embryologists were fascinated by the fact that when an embryo is divided into two halves perfectly patterned twins can form. Fortunately, this vocation of embryology towards studying the whole is now paying off and we are beginning to understand how cells can communicate with each other over long distances spanning hundreds of cells.
In this essay I will examine how complexity can be deciphered in animal development. Three issues will be addressed:
First, in biology physiological mechanisms are determined by the historical twists and turns a species had to endure in order to survive during its evolution. Although humans share a common gene tool-kit with other animals, each species found unique solutions to the obstacles presented by the selective pressures of natural selection. Consequently, in biology it is difficult to uncover invariant universal laws that can be expressed as mathematical equations.
Second, we will examine how the researcher usually constructs a mental model of how the mechanism might work, and then tests it by experiment. To be productive, a hypothesis must be testable using the techniques available in that particular period. Consequently, our level of understanding changes over time. The new ability to readily sequence and clone DNA through molecular biology opened the door for great advances in recent decades.
Finally, the main part of this essay will describe how this step-by-step approach of simple hypotheses tested by experiment allowed the deciphering of one of most complex regulatory systems imaginable, the molecular mechanism of induction of an invariant pattern of cell differentiation in the early vertebrate embryo. We will examine how a network of extracellular proteins that generates a dorsal-ventral (D-V) morphogenetic gradient of growth factor signaling was discovered. This system has the remarkably capacity of regenerating a perfect pattern after the morphogen gradient is perturbed. This property of self-regulation is one of the most fascinating phenomena in living organisms, which could start to be addressed from a physico-chemical perspective only after recombinant DNA technology made possible the purification of the proteins involved in the network.
1 Living organisms are shaped by their evolutionary history
Physics and chemistry obey universal laws that can be expressed by mathematical equations. These laws have remained unchanged since the beginning of the universe. Life on earth has a single origin, as revealed by the fact that all living organisms share the same basic genetic code as the one in human DNA (Collins, 2006). Recent advances in DNA sequencing have allowed entire genomes to be sequenced readily. Bioinformatic “systems biology” approaches allowed scientists to describe the expression of thousands of genes in many cell types. Computational analyses of DNA sequences led to detailed catalogues of functional sites in humans. An ENCyclopedia of DNA Elements (ENCODE) in humans was recently published in a paper with almost 450 co-authors (Dunham et al., 2012); it provides one of the best examples of current “big science” efforts. Computers are very efficient at comparing linear sequences of A, G, T and C. However, despite these extensive computer analyses of the complexity of the human genome we are still very far from understanding the principles by which the genotype is converted into phenotype. We know the sequence of nucleotides, but do not understand how the genetic program is interpreted to produce a perfect organism generation after generation.
The main obstacle to revealing general laws in biology is the historical nature of life. Animals are shaped by evolution. To survive the strictures of natural selection a species acquires mutations, duplications, and gene losses that record within its DNA its previous history (Gould, 2002; De Robertis 2008). For this reason it is very difficult to uncover invariant laws beyond those of physics and chemistry. Nevertheless, extraordinary advances have been achieved using the experimental method. The main goal of this paper is to illustrate how the enormous complexity of embryonic development can be interrogated productively using an hypothesis-driven approach.
2 Conceiving hypotheses that can be tested by experiment
How is new knowledge acquired in biology? The best description I know of the mental process involved is presented in an old book by French physiologist Claude Bernard (1865). Entitled “Introduction to the Study of Experimental Medicine”, its lessons are still valid today. Bernard was a noted physiologist, who discovered that the function of the liver and other organs was to deliver internal secretions into the blood. He also proposed that “animals have really two environments: a milieu extérieur in which the organism is situated, and a milieu intérieur in which the tissue elements live”, and that physiological mechanisms were directed at maintaining the milieu intérieur constant.
The leitmotif of C. Bernard’s book on the epistemology of experimental biology is that discovery starts with an a priori idea or hypothesis of how a phenomenon might occur. Using our reason we then devise experiments that might support or falsify the hypothesis and finally subject the hypothesis to proof by experiment. “The true scientist is one whose work includes both experimental theory and experimental practice. (1) He notes a fact; (2) à propos of this fact an idea is born in his mind; (3) in the light of this idea, he reasons, devises an experiment, imagines and brings to pass its material conditions; (4) from this experiment, new phenomena result which must be observed, and so on and so forth.” In modern terms we call this approach hypothesis-driven research. To conceive an experiment we first need an idea or mental picture of how the process might work. “The experimental idea is the result of a sort of presentiment of the mind which thinks things will happen in a certain way. In this connection we may say we have in our minds an intuition or feeling, as to the laws of nature, but do not know their form. We can learn it only from experiment.” In general, the new hypothesis is based on previous knowledge that pointed our attention to areas that remained unexplained.
The experiment puts the question to the natural world, which gives an answer independent of our own prejudices. At this point, the observer evaluates the results without preconceptions and decides whether the experimental hypothesis is verified, disproved, or suggests an alternative hypothesis. The key to the experimental method is that we must accept the result offered by nature and not replace it with our own reason. “Experimenters must doubt their intuition, i.e., the a priori idea or the theory which serves as their starting point; this is why it is an absolute principle always to submit one’s idea to the experimental criterion so as to test its value.” In my experience, when an idea is confirmed by experiment it frequently opens doors for new discoveries. If the hypothesis is incorrect, we rapidly encounter a wall of repeated obstacles and the verdict becomes evident. At some point even a productive experiment ceases to yield new insights; this indicates that new theories or methods will be required to advance further.
The story of the molecular dissection of the dorsal-ventral morphogen gradient provides an example right out of Claude Bernard’s book. Experiments on the self-regulation of embryonic development suggested ideas that changed over time as new technical advances allowed deeper levels of understanding of development at a physico-chemical level.
3. Unraveling the induction of embryonic tissue differentiation
3.1 Self-regulation in development
One of the most remarkable properties of embryos is that they are able to regenerate missing parts. After experimental manipulations the embryo attempts to self-regulate towards the whole. This property ensures that the most perfect embryo possible is produced time after time despite variations in egg size or environmental changes such as temperature. The experimental approach in embryology started in 1883 when Roux killed one of two cells of a frog egg with a hot needle and found that the surviving half gave rise to a partial embryo. However, in 1891 Driesch separated the two first cells (called blastomeres) of a sea urchin embryo and found that each was able to form a complete, although smaller, embryo.
Amphibian embryos provide a very good material for these experiments because the dorsal (future back) and ventral (future belly) sides can be distinguished from each other. Even before the first cell division, the future dorsal side can be recognized by a less pigmented dorsal crescent (resulting from a rotation of the egg cytoplasm along microtubules). In 1901 Hans Spemann used a baby hair loop to constrict salamander embryos into two halves. Much later, we found that the embryo can self-regulate a perfect pattern even after bisection with a scalpel blade at the late blastula (9000-cell) stage (for a timeline article on experimental embryology see De Robertis, 2006). In modern times researchers use the South African frog Xenopus laevis (Gurdon and Hopwood, 2000), which provides large numbers of eggs all year long.
If the embryo fragments contain both dorsal and ventral components identical twins can result. This provides one of the most extreme examples of regeneration since the entire missing half of the body, with all its organs, is perfectly replaced. The resulting embryos are “scaled” so that they are smaller in size but perfectly patterned. If the half embryo contains only ventral cells a spherical “belly piece” consisting of ventral tissues such as epidermis and blood is produced (Spemann, 1928). However, the dorsal half of the embryo is able to scale, forming a smaller but well-proportioned embryo containing dorsal tissues such as the central nervous system (CNS) and notochord, as well as ventral tissues. These experiments suggested that the dorsal side contained an activity able to organize embryonic tissue differentiation.
The key experiment for understanding embryonic patterning was performed by Spemann’s graduate student Hilde Mangold, who transplanted a small region of the gastrula (10,000-cell stage) called the dorsal lip of the blastopore into the ventral side of a host embryo (Spemann and Mangold, 1924). The blastopore is the region through which the cells that will form the endoderm and mesoderm layers of the embryo invaginate. The cells that remain on the outside form the third germ layer, the ectoderm. A small grafted dorsal region had a very potent effect on the embryo, dividing the pattern of the whole into two Siamese twins:
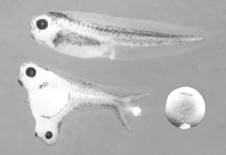 |
Figure 1. The Spemann experiment: transplantation of a small fragment of dorsal tissue from the dorsal lip can induce twinning. Note the less pigmented grafted tissue in the ventral side of an early Xenopus embryo. |
The tissue used by Hilde Mangold for the transplantation was from an unpigmented species of salamander, so that the lineage of the grafted cells could be distinguished from the pigmented host. The transplanted cells differentiated into notochord and induced neighboring cells to differentiate into somite (the embryonic tissue segments that give rise to skeletal muscle, bones and connective tissue of the dermis) and kidney in the mesoderm, and brain and spinal cord in the ectoderm. This was the crucial experiment that demonstrated that during development groups of cells can direct the differentiation of other cells. Spemann called the dorsal lip tissue the “organizing center” of the embryo. His thinking borrowed heavily from physics, which was the dominant science at this time. From electromagnetism he borrowed the term embryonic “induction” to designate the ability of organizer tissue to change cell differentiation and considered that cells in the embryo formed a morphogenetic “field” that was capable of self-regulation.
The organizer experiment represented the highest point of experimental embryology and Spemann received the Nobel Prize for Medicine or Physiology in 1935 for the discovery of embryonic induction. This experiment firmly established that the dorsal side of the embryo had inductive abilities and that organizer tissue played a crucial role in self-regulation. A molecular analysis would have to wait for many decades until gene cloning made molecular embryology a practical possibility. However, a new working hypothesis had been formulated by the Spemann-Mangold experiment: that dorsal organizer tissue secretes signals that induce cell differentiation at a distance.
3.2 Morphogen gradients
Another important advance took place in the 1950s. It was not an experimental advance but rather a theoretical one. Mathematician Alan Turing proposed a simple yet powerful theory to explain the behavior of differentiating biological systems. He suggested that anatomical structure might result from the diffusion of hypothetical substances that he called morphogens. He took the complex embryonic system and rendered it much simpler. Turing realized that simple physico-chemical laws were able to explain many of the facts of embryonic morphogenesis. He imagined that a system of chemical substances capable of reacting with each other and diffusing through a tissue would be able to generate pattern (Turing, 1952). “The systems actually to be considered consist therefore of masses that are not growing, but within which certain substances are reacting chemically, and through which they are diffusing. These substances will be called morphogens, the word being intended to convey the idea of a form producer.” The D-V patterning system that was later uncovered in the frog Xenopus indeed contained many interacting protein molecules, which are able to diffuse over long distances in the embryo.
The reactions between morphogens depended of their concentration (law of mass action) and on their diffusion according to Fick’s law of diffusion in fluid medium. Turing formulated a general partial differential equation to describe quantitatively the changes in concentration of a morphogen (C) over time (δt):
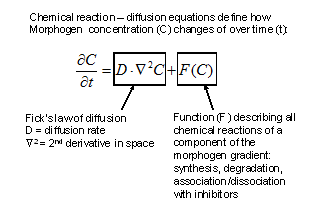 |
Figure 2. Evolution of morphogen (C) concentration in time and space. |
The right side of the equation describes that, following Fick’s law of diffusion, the change in concentration of the morphogen (C) is proportional to its diffusion rate (D) and to the second derivative in space of the morphogen concentration (Ñ2C). In addition, the change in morphogen concentration is also a function (F) of all the chemical reactions it undergoes (such as synthesis, degradation, and association and dissociation with other proteins such as antagonists). From this initial insight a large number of “reaction-diffusion” computer models to explain the behavior of developing systems have been derived (reviewed in Meinhardt, 2008; Plouhinec and De Robertis, 2009).
A further advance was the realization by Gierer and Meinhardt (1972) that in theory a pair of morphogens composed of an Activator and an Inhibitor originating from the same cells can generate stable patterns:
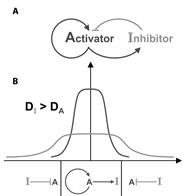 |
Figure 3. An Activator/Inhibitor pair of interacting morphogens. |
The system shown in the figure consists of two diffusible morphogens secreted by the same source. In panel A we see that the Activator turns on its own production and also the synthesis of an Inhibitor that interacts with the Activator. In panel B we see how a field of cells can be patterned into two different zones provided the inhibitor diffuses faster than the activator. Activator (A) and Inhibitor (I) are secreted by the source at the center and turned off by a preponderance of inhibitor in the periphery. Such pairs of Activators and Inhibitors were found decades later to play prominent role in maintaining the dorsal and ventral signaling centers present in the frog embryo.
It is remarkable that these mathematicians could provide a theoretical framework for understanding long-range diffusion-reaction of morphogens at a time when the chemical nature of not even a single morphogen was known. A new working hypothesis was formulated, that morphogens would form gradients of signaling activity that could cause the differentiation of different cell types at different thresholds of activity. Further, these morphogens would be expected to react with each other, an idea that was fully vindicated by work on the D-V patterning system. Respecification of an embryonic morphogen gradient by the organizer graft could explain the amazing Siamese twins observed in the Spemann transplantation experiment
3.3 A Dorsal-Ventral gradient of BMP signaling controls histotypic differentiation
D-V patterning results from a gradient of activity of a family of secreted growth factors called Bone Morphogenetic Proteins (BMPs). The first correlation between tissue differentiation and BMPs came from the study of Drosophila mutations in a gene called decapentaplegic (dpp), which is the homolog of vertebrate BMP2/4 (Ferguson and Anderson, 1992). In mammals, BMPs had a long history of being involved in bone differentiation. It was first noted that bone fragments transplanted subcutaneously or intramuscularly in rabbits could induce bone differentiation even after all cells had been killed with ethanol (Levander, 1938). Marshall Urist, an orthopedic surgeon working at the University of California, Los Angeles, found that the proteinaceous bone extracellular matrix, obtained by removing Calcium from bones by soaking them in Hydrochloric acid (HCl) for several days, had potent ectopic bone morphogenetic activity after transplantation into rabbits or rats (Urist, 1965). The active proteins were purified and cloned by a biotechnology company, and found to correspond to growth factors designated BMP2 to BMP7 (Wozney et al., 1988).
Growth factors are proteins that are secreted into the extracellular space, where they bind to surface receptors in other cells, triggering changes in cell signaling. They provide the main mechanism by which cells communicate with each other. BMP2 to BMP7 belong to the larger Transforming Growth Factor-beta (TGF-β) family of growth factors, which consists of 30 different genes in humans. Although first discovered because of their bone morphogenetic properties in mammals, BMPs play a central role in D-V embryonic patterning.
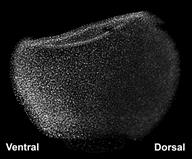 |
Figure 4. D-V gradient of BMP signaling revealed by phospho-Smad1 in Xenopus. |
In zebrafish and Xenopus embryos it is possible to visualize the gradient of BMP signaling activity. This is done indirectly, by staining whole-mount embryos with an antibody specifically directed against the phosphorylated form of the Smad1 transcription factor. In Figure 4 one can see that BMP activity is lowest on the dorsal side and gradually increases towards the ventral side, in which the cell nuclei accumulate higher amounts of phospho-Smad1.
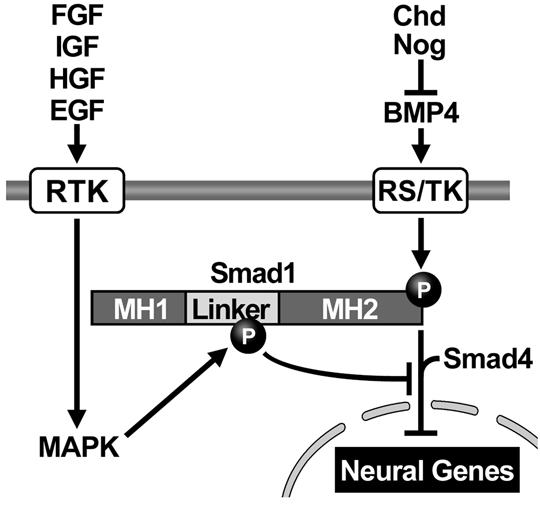
BMPs (or other members of the TGF-β superfamily) bind to two receptors on the cell surface, which become activated. The BMP receptors are transmembrane proteins containing a cytoplasmic enzymatic domain able to phosphorylate hydroxyl groups of Serine or Threonine amino acids in proteins. Their main target is a transcription factor called Smad1, which becomes phosphorylated at its carboxy-terminal end. Phospho-Smad1 (pSmad1) binds a second protein called Smad4 (also known as Deleted in Pancreatic Carcinoma). These two proteins together translocate inside the nucleus where they bind to DNA. Smad1/4 do not have a very high DNA-binding affinity and therefore require additional partner transcription factors bound to nearby DNA sites in order to activate or repress gene activity (Massagué, 2000). BMP/TGF-β can activate different genes in different germ layers because these differ in the types of partner transcription factors they contain, according to their previous developmental history. This process, by which a protein bound to the outside membrane of the cell can change the activity of genes in the nucleus, is called signal transduction.
3.4. Cloning the Spemann organizer genes.
When molecular biology, the great equalizer of modern biology, became practical the search for the signals produced by the dorsal organizer tissue that mediate embryonic induction was on. In our laboratory a gene library from manually-dissected dorsal blastopore lips from Xenopus was prepared. We succeeded in isolating a homeobox gene designated goosecoid (Cho et al., 1991). This gene allowed us to visualize Spemann’s organizer using in situ hybridizations to its mRNA. Previously, the existence of organizer tissue had to be deduced from its effects after transplantation experiments.
Overexpression of goosecoid mRNA was able to induce twinned axes. However, goosecoid encodes an intracellular DNA-binding protein and we knew from Spemann’s experiments that the factors responsible for embryonic induction had to be diffusible over long distances. This suggested the hypothesis that goosecoid might activate the synthesis of secreted proteins.
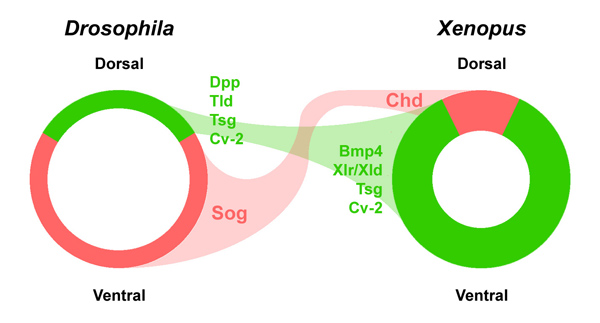
In 1994 we were able to isolate chordin, a gene activated by goosecoid from our Xenopus organizer library (Sasai et al., 1994). Richard Harland had also isolated noggin (Smith and Harland, 1992) and Douglas Melton follistatin (Hemmati-Brivanlou et al., 1994). It was later found that all three gene products were able to inhibit BMP signaling (Sasai et al., 1995). The dorsal side of the gastrula embryo secretes BMP antagonists and the ventral side BMP4 and BMP7:
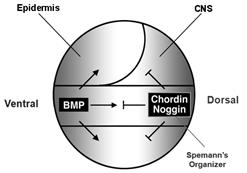 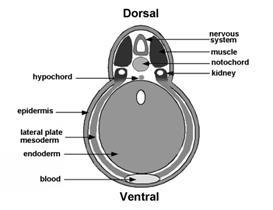 |
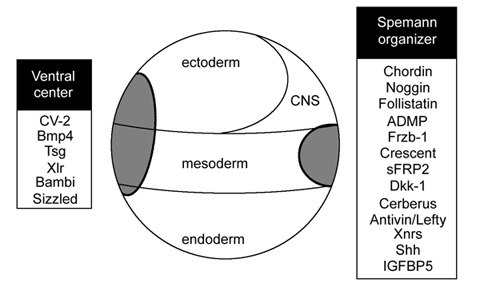
Figure 5. BMP antagonism patterns the three germ layers, leading to the stereotypical arrangement of tissue differentiation in the vertebrates. |
Our initial hypothesis was that dorsal tissue would be the source of novel growth factors. Spemann’s organizer proved a fertile fishing ground for new molecules, but what was found instead was that it secreted a large number of secreted growth factor inhibitors (reviewed in De Robertis and Kuroda, 2004).
Curiously, BMP signaling levels were able to regulate cell differentiation in the ectoderm, mesoderm and endoderm layers simultaneously. Explants of future ectoderm (cells from the animal cap of the embryo) differentiate into CNS at low BMP levels (e. g., in the presence of BMP antagonists) and into epidermis when BMP signaling levels are high. Similarly, in the mesoderm low BMP gives rise to notochord (a flexible rod used by chordate embryos for swimming), at slightly higher levels skeletal muscle (arranged in repeated segmental structures called somites), then kidney (each embryonic segment develops a kidney tubule), then lateral plate (which gives rise to the body wall) and at the highest BMP levels blood islands (Figure 5). These tissues represent the invariant body plan shared by all vertebrates. This then raises the question of how many morphogen gradients exist. Is there one gradient per germ layer? How would each gradient be regulated coordinately so that a perfectly harmonious embryo is formed?
The embryo has only one chance to allocate these tissues perfectly so it is not surprising that the BMP gradient is tightly regulated. While there are many growth factor antagonists secreted by Spemann’s organizer cells, Chordin proved the most informative for the regulation of the D-V signaling gradient; it was at the heart of the organizer phenomenon.
3.5 Chordin regulates the D-V BMP gradient
When Chordin is microinjected into a ventral cell (in the form of synthetic mRNA prepared in the test tube) it recapitulates the organizer experiment, forming a second neural tube, somites and even a second gut cavity. Chordin can be depleted in Xenopus embryos using microinjected Morpholino oligos (MO). These antisense reagents (which resemble RNA but have a morpholine ring replacing the ribose, so they not degraded easily by cellular enzymes) hybridize to mRNA and prevent its translation into protein. Depletion of Chordin produced embryos that developed with smaller heads and dorsal tissues and enlarged ventral structures. However, an embryonic axis was still formed. Later, Harland found that the combined depletion of Chordin, Noggin and Follistatin led to a catastrophic loss of all dorsal tissues (Khokha et al, 2005). Similarly, in the mouse double knockout of Chordin and Noggin the forebrain, midbrain and notochord and are lost (Bachiller et al., 2000). Thus, the BMP antagonists secreted by organizer tissue are able to compensate for each other.
Chordin is absolutely essential for the activity of Spemann organizer grafts. An organizer transplanted to the ventral side of the gastrula invaginates inside the embryo, inducing new dorsal tissues. This is particularly clear when a pigmented graft is placed in an albino embryo. When the organizer was depleted of Chordin it remained as a patch of epidermis that was completely devoid of inducing activity (Oelgeschlager et al., 2003). Transplantation is a very powerful tool in biology. In this case, when dorsal cells were challenged by placing them in a new ventral surrounding their diminished biological capacity due to the loss of the Chordin gene product was strikingly revealed.
3.6 Chordin is used throughout the animal kingdom
We realized that Chordin had to be a very important molecule very early on. A gene of similar sequence was cloned in the fruit fly Drosophila called short gastrulation (sog). We collaborated with F. Michael Hoffman and Edwin “Chip” Ferguson to show that microinjected chordin and sog mRNA induced neural tissue both in Drosophila and frog embryos (Holley et al., 1995). This led to the realization that we had discovered an ancient molecule that had been conserved in evolution between fly and amphibian embryos.
Extensive genetic screens in the zebrafish Danio rerio by Christiane Nüsslein-Volhard supported the discoveries from our work in Xenopus described below. In a satisfying convergence, zebrafish loss-of-function mutations that affected the allocation of dorsal-ventral tissues were found to affect genes in the Chordin pathway. Loss-of-function mutations that increased ventral tissues eventually corresponded to Chordin and Sizzled, and mutations increasing dorsal tissues corresponded to BMP7, BMP2b, a BMP receptor, Smad5 and Tolloid (Little and Mullins, 2006).
These genetic findings greatly increased confidence that we had uncovered a patterning mechanism that was generally applicable across animal embryos. Importantly, the effects of sog mutations were known to be enhanced when the number of dpp/BMP4 genes was increased in Drosophila (Ferguson and Anderson, 1992). This led us to the new hypothesis that Chordin and BMPs worked on a common signaling pathway.
3.7 Chordin is part of a biochemical pathway
Chordin mRNA is expressed at high levels in dorsal cells in the exact region that has inducing activity after transplantation. We measured the amount of Chordin secreted by the frog embryo during gastrulation and found that it is produced in prodigious amounts. If distributed uniformly in the extracellular space, Chordin protein would reach concentrations of 33 nanomolar (nM). In the dorsal side, where it is produced, Chordin must reach much higher extracellular concentrations. BMP concentrations have not been measured in embryos, but in other tissues they are in the picomolar (pM) range. Thus, a vast excess of Chordin exists during early development.
Since Chordin is a BMP antagonist, in principle its localized expression in the dorsal side could be sufficient to account for the BMP signaling gradient, even if BMP expression were uniform. However, our investigations discovered that Chordin is part of a biochemical network of extracellular proteins that encompasses the entire embryo. It gradually became clear that the organizer effect is not due only to the action of the dorsal side but also to the reaction of ventral cells.
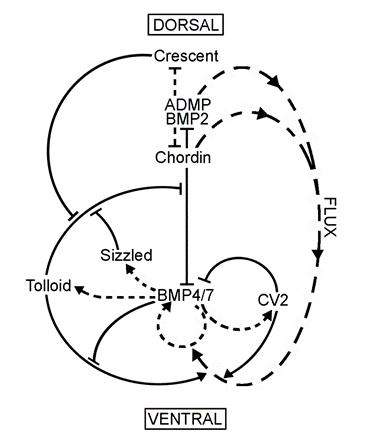 |
Figure 6. The extracellular biochemical network of interacting proteins that explains self-regulation of D-V patterning. |
We found that several secreted proteins are synthesized by the same cellular sources. The embryo has a dorsal and a ventral center that communicate through secreted proteins that interact with each other (indicated by solid lines). Dorsal genes are expressed when BMP levels are low, while ventral genes are expressed when BMP levels are high (transcriptional activity is indicated by lines with short stipples). The two centers adapt to changes in signaling because all the components of the system are under opposing transcriptional control by BMP. The long stippled arrows indicate the flux or flow of Chordin bound to dorsal BMPs, BMP2 and ADMP, towards the ventral side. (ADMP stands for anti-dorsalizing morphogenetic protein, Moos et al., 1995.) The function of each of the extracellular proteins of this pathway was determined by a combination of biochemical and embryological experiments, as explained below.
3.8 Chordin is a BMP antagonist regulated by a metalloproteinase
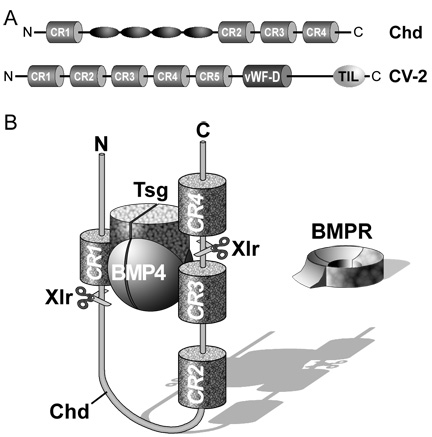
Chordin encodes a large protein containing four Cysteine-rich (CR) domains that serve as BMP-binding modules. It has a cofactor called Twisted gastrulation (Tsg) that helps keep BMPs soluble and binds to both Chordin and BMPs. The ternary complex of Chordin, BMPs and Tsg prevents binding of BMPs to BMP receptors on the cell surface and in this way inhibits BMP signaling.
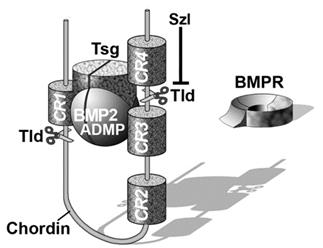 |
Figure 7. The ternary complex of Chordin (Chd), BMP4 and Tsg inhibits binding to BMP receptor (BMPR). |
Purified Chordin binds BMPs with an affinity (dissociation constant, KD) in the low nM range (Piccolo et al., 1996). However, the inhibitory action of Chordin is not permanent and can be reversed by metalloproteinases of the Tolloid family. Tolloid was identified in classical Drosophila genetic screens (Wieschaus and Nüsslein-Volhard, 1980) as a gene that increased Dpp/BMP signaling. In collaboration with Leslie Dale, we showed that the metalloproteinase Xolloid-related (indicated as Tld and represented by scissors in Figure 7) was able to cleave Chordin at two precise sites. When this happens, the affinity of the cleaved Chordin for BMP decreases precipitously and the complex of BMP and Tsg is able to bind to BMP receptors, restoring signaling (Piccolo et al. 1997). Because Tolloid/Xlr is expressed in the ventral side, it serves as a ventral sink that degrades Chordin originating from Spemann’s organizer, allowing the flux of BMPs from more dorsal regions to the ventral side in which BMP signaling is maximal. Activity of the Tolloid protease is the rate-limiting step in D-V patterning and is subjected to stringent regulation.
3.9 Sizzled and Crescent are inhibitors of Tolloid activity
Sizzled is a ventral center molecule that is expressed at high BMP signaling levels (Collavin and Kirschner, 2003). In zebrafish the Sizzled mutation (called ogon/mercedes) had a phenotype intriguingly similar to that of Chordin mutants (Yabe et al., 2003). We microinjected sizzled mRNA into dorsal or ventral half-embryos in Xenopus and noted that it had strong dorsalizing effects on dorsal fragments (expansion of the CNS) but none at all in ventral fragments. Thus, sizzled function required a dorsal component, giving rise to the idea that it might inhibit the degradation of Chordin by Tolloid.
Sizzled encodes a secreted Frizzled-related protein (sFRP). This class of protein is normally involved in inhibiting Wnt signaling. However, in the case of Sizzled the protein has evolved so that it is able to bind to the Tolloid proteolytic site, but is unable to be cleaved by this enzyme. In this way, Sizzled acts as a competitive inhibitor of Tolloid. Like most components in the pathway shown in Figure 6, the Sizzled/Tolloid binding has an affinity in the 20 nM range (Lee et al., 2006).
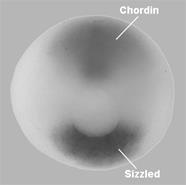 |
Figure 8. Sizzled is expressed in the ventral side and Chordin in the dorsal organizer at the circular blastopore stage (the blastopore will later become the anus). |
Sizzled depletion by antisense morpholinos results in the same phenotype as Chordin loss-of-function, because in its absence the activity of Tolloid increases and Chordin is degraded. When overexpressed Sizzled has an anti-BMP effect because it inhibits Tolloid, causing the accumulation of Chordin. The increase in Chordin levels then inhibits BMP signaling. On the dorsal side of the embryo, a protein structurally related to Sizzled called Crescent also serves as a Tolloid inhibitor, but under the opposite transcriptional regulation (Figure 6; Ploper et al., 2011).
Sizzled acts as a feedback inhibitor of Tolloid and is expressed in copious amounts in the Xenopus embryo. Like Chordin, it would be present at concentrations of 30 nM if distributed uniformly in the extracellular space (Lee et al., 2006). Tolloid and Sizzled behave as an Activator/Inhibitor pair in the sense described in Figure 3. Tolloid will activate its own synthesis by increasing BMP signaling, while Sizzled will inhibit Tolloid activity competitively.
Tolloid activity is also regulated by direct binding of BMP to protein domains outside its catalytic region. If BMP levels become high it binds to domains in Tolloid called CUB domains, inhibiting enzyme activity in a non-competitive fashion (Lee et al., 2009). This new negative feedback loop provided a molecular explanation for an old mystery in the field. When the first peptide sequences were obtained from extracts with bone-inducing activity (Wozney et al., 1988) the first protein identified, designated BMP1, had the sequence of a Tolloid enzyme containing three CUB domains. The reason why BMP1 was purified together with the BMP2 to 7 growth factors was simple: Tolloids are BMP-binding proteins. In conclusion, Tolloid activity is highly regulated and plays a key role in the communication between the dorsal and ventral sides of the embryo.
3.10 Crossveinless 2 concentrates Chordin/BMP complexes in the ventral side
Another component of the Chordin/BMP pathway is Crossveinless 2 (CV2). We first identified it by searching sequencing databases for vertebrate genes that contained CR domains similar to the BMP-binding modules of Chordin. Once the complete sequence was obtained, it became clear that it was homologous to a gene previously described in Drosophila called Crossveinless 2 (Conley et al., 2000). In the fly wing, CV2 is required to reach the maximal BMP signaling required for formation of cross vein structures. Like Chordin, overexpressed CV2 can act as a BMP antagonist through its BMP-binding modules. CV2 expression is activated by high BMP levels and is therefore produced in the ventral center. When one depletes Chordin, CV2 expression increases, compensating for the loss of Chordin from the opposite side of the embryo. When both Chordin and CV2 are depleted, ventral tissues are greatly expanded (Ambrosio et al., 2008). One difference with Chordin is that CV2, although it is a secreted protein, is not able to diffuse through the extracellular space because it remains anchored by glycosylated proteins (called glypicans) to the surface of the cells that secrete it (Serpe et al., 2008).
Why does CV2 have pro-BMP effects in Drosophila wing? We addressed this by asking whether CV2 interacted with other components of the D-V patterning system. Using a biochemical approach we found that CV2 has a second activity. It binds with considerable affinity (dissociation constant 1.4 nM) to Chordin and with even higher affinity to Chordin/BMP complexes. In the frog embryo, CV2 acts as a molecular sink concentrating Chordin/BMP complexes in the ventral side. Once there, Tolloid proteases can cleave Chordin, allowing BMP to signal through its receptors. In this way BMP signaling is boosted on the ventral side by the combined action of CV2 and Tolloid, which facilitate the flux of BMPs produced in more dorsal regions of the embryo and transported by Chordin.
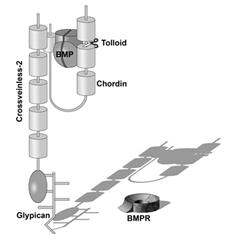 |
Figure 9. Crossveinless 2 (CV2) serves as a molecular sink that concentrates Chordin/BMP complexes on the ventral side, where BMPs are then released by cleavage of Chordin by Tolloid. CV2 does not diffuse far from the cells where it is produced because it binds to glypicans on the cell surface. |
Starting with the isolation of Chordin we were able to identify, one at the time, multiple components that react molecularly in this morphogenetic pathway that comprises the entire embryo. At the stage being studied, the embryo is composed of 10,000 cells, raising the question of how the information in the dorsal and ventral centers is transmitted long range to produce a self-regulating morphogen gradient. We address this next.
3.11 Opposite transcriptional regulation of D-V molecules is the key to self-regulation
The dorsal and ventral centers are under opposite transcriptional control by the BMP signaling pathway. Dorsal center molecules are produced when BMP levels are low while ventral molecules are secreted when BMP signaling is high (Figure 6, stippled arrows). Interestingly, the dorsal and ventral poles of the embryo express proteins of similar biochemical activities but under the opposite regulation.
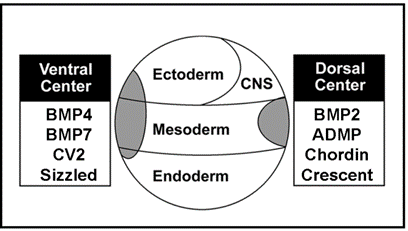 |
Figure 10. Proteins of similar structure and function in the ventral and dorsal sides, but under opposite transcriptional control. |
The dorsal side produces BMP2 and ADMP, while the ventral side secretes BMP4 and BMP7. All have similar activities, activating BMP receptors that phosphorylate Smad1. Chordin is secreted by Spemann’s organizer while on the ventral side CV2, which contains similar BMP-binding modules, is produced. Similarly, the ventral center secretes Sizzled and the dorsal side the closely related molecule Crescent. These molecules can compensate for each other from different poles of the embryo. For each action of Spemann’s organizer there is a reaction in the ventral side of the embryo.
The self-adjusting nature of this system is illustrated by an experiment in which the cavity of blastula embryos (called the blastocoele) was injected with Chordin protein to decrease BMP signaling or with BMP4 protein to increase signaling. When BMP levels were decreased, expression of ADMP mRNA went up, increasing BMP signaling. When BMP4 was injected, Sizzled transcription went up, indirectly dampening BMP signaling levels (via the inhibition of Tolloid, causing an increase in Chordin that antagonizes BMP). The embryo behaves as a molecular seesaw, forming a self-adjusting BMP gradient (Reversade and De Robertis, 2005).
3.12 BMPs and self-regulation
The Xenopus embryo expresses four main BMPs: ADMP, BMP2, BMP4 and BMP7. When we depleted embryos of BMP2, 4 and 7, we found that ventral half-embryos differentiated profuse amounts of dorsal tissues such as CNS, but dorsal half-embryos scaled to an almost normal embryonic pattern. This suggested that the organizer, which is the region of lowest BMP signaling, also contained a ventralizing signal.
This led us to formulate the hypothesis that ADMP was produced on the dorsal side but its activity blocked by the presence of Chordin secreted by the same cells. Indeed, when the four BMPs were depleted a spectacular transformation was observed, in which the entire ectoderm of the embryo became converted into CNS tissue (Reversade and De Robertis, 2005). Embryos were pear-shaped (radial) and covered mostly by forebrain, with a small amount of spinal cord near the blastopore.
The availability of these radial embryos covered in neural tissue gave us a new experimental opportunity. Would wild-type tissue be able to secrete BMPs in sufficient amounts to restore epidermal differentiation at a distance? Transplanted ventral tissue was able to restore D-V pattern and epidermal differentiation, making the important point that a long-distance ventral signaling center exists. An even more interesting result was obtained by transplanting lineage-traced wild type dorsal grafts into BMP depleted embryos. The grafted gave rise to notochord and restored D-V patterning. Epidermis was formed in embryos that otherwise would have developed only neural tissue in the ectoderm. Importantly, there was no epidermal induction near the dorsal graft, which was the only source of BMP in these embryos. Epidermis differentiated on the ventral side at a great distance from the transplant. This suggested that ADMP and BMP2 from the organizer grafts diffused, presumably bound to Chordin and unable to signal, and were released for signaling when Chordin is cleaved by Tolloid enzyme in the ventral side (Reversade and De Robertis, 2005). This was our best, although indirect, evidence for long-distance flux of morphogens in Xenopus for many years.
3.13 Chordin forms a gradient in the space that separates ectoderm from endomesoderm
We have recently been able to visualize Chordin protein by immunohistochemistry in the Xenopus gastrula. This required technical improvements since amphibian embryos have autofluorescent yolk particles. Chordin mRNA is produced in 60o of arc of the dorsal side. However, Chordin protein was present as a gradient extending from the dorsal side to the ventral-most part of the embryo. The staining was specific because it was eliminated by depletion of Chordin. The unexpected surprise was that Chordin does not diffuse randomly in the space between cells but instead is found specifically in the extracellular space that separates the ectoderm from the endomesoderm.
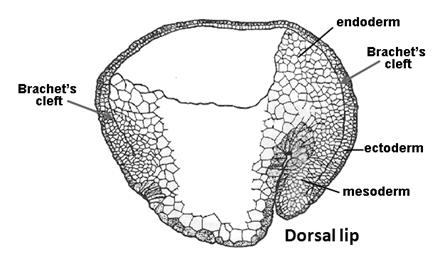 |
Figure 11. Brachet’s cleft separates ectoderm and endomesoderm (modified from Nieuwkoop and Florschütz, 1950). |
In amphibian embryos this virtual cavity is called Brachet’s cleft (in honor of Albert Brachet, the distinguished Belgian embryologist). All vertebrate embryos have an extracellular matrix containing Fibronectin and other proteins separating ectoderm and mesoderm. Therefore, Brachet’s cleft is not an amphibian-specific structure. Confocal optical sections revealed a smooth gradient of Chordin extending from the organizer to the ventral side through this space. From Brachet’s cleft Chordin protein can be seen diffusing into both the ectoderm and the mesoderm. Within this narrow cleft, Chordin behaves as predicted by our biochemical pathway. For example, when Tolloids are depleted with antisense morpholinos nuclear phospho-Smad1 (a measure of BMP signaling) decreases while Chordin protein accumulates to high levels in Brachet’s cleft. This increase in Chordin protein is particularly obvious in the ventral cleft. These experiments directly demonstrate the long-range diffusion of the Chordin morphogen in the gastrula embryo.
It appears that the embryo has a single gradient of Chordin that can pattern all three germ layers at the gastrula stage. This provides a simple solution to the question of how many BMP gradients exist: only one. As mentioned above, Chordin reaches high concentrations in the embryo. The fact that it diffuses through a narrow region suggests that Chordin, and probably other components of the D-V patterning pathway, must reach very high concentrations in the space between ectoderm and endomesoderm. During gastrulation the germ layers undergo extensive morphogenetic movements; it is possible that cells might read the Chordin/BMP gradient contained in this extracellular matrix as they move along its surface.
The most intriguing property of the D-V patterning system is its ability to self-regulate. The ability to visualize gradients allowed us to examine embryos bisected in D-V fragments (unpublished results). A phospho-Smad1 gradient was re-formed on dorsal halves, while in the ventral half, where BMP signaling is not opposed by antagonists, very high uniform levels of BMP signaling were found. Using Chordin antibodies, the Chordin gradient in Brachet’s cleft was re-formed in dorsal halves, but not in ventral ones. Thus, the BMP/Chordin gradient is able to rescale pattern after experimental perturbation of the system, opening new opportunities for investigating the mechanisms of D-V cell differentiation.
Conclusions
The considerable complexity of the D-V patterning biochemical pathway was deciphered using the hypothesis-driven experimental approach outlined by Claude Bernard (1865). The investigator formulates an idea in his mind, reasons an experiment that might prove it or disprove it, allows the experiment to give an objective answer, and finally designs new hypotheses based on the observed results. Embryologists were always fascinated with the self-regulating properties of embryos. Spemann found that dorsal organizer tissue could induce cell differentiation over long distances. Mathematicians proposed that the long range diffusion of morphogens according to the laws of physics could account for biological phenomena and proposed that morphogens reacting with each other could generate stable patterns provided they arise from the same source and have different diffusion rates.
With the background of these hypotheses, a large number of extracellular proteins that mediate dorsal-ventral pattern were isolated from the frog embryo. Key supporting insights came from Drosophila and zebrafish genetics. Using a combination of biochemistry with purified proteins and embryological studies involving the simultaneous depletion of multiple gene products with morpholinos in combination with transplantation experiments, it was possible to construct the biochemical pathway shown in Figure 6. All its components are secreted proteins that are able to directly interact with each other, forming negative feedback loops of activators and inhibitors synthesized by cells in the dorsal and ventral poles of the embryo.
Dorsal genes are expressed when BMP signaling is low and ventral genes when it is high. The genes that are turned on and off also interact with each other at the protein level. The D-V gradient is adjusted redundantly by regulating the synthesis of its components, by direct protein-protein interactions between morphogens, and by diffusion. The entire embryo participates in maintaining the D-V BMP gradient, so that for each action in the dorsal side there is a reaction in the ventral side. The dorsal and ventral centers are able to communicate with each other through the flux of Chordin, a protein that transports BMPs ventrally, and through the metalloproteinase Tolloid that cleaves Chordin. A gradient of Chordin is formed in the extracellular matrix that separates ectoderm from endomesoderm. It appears likely that a single gradient of Chordin/BMP provides patterning information for all germ layers. The Chordin/BMP pathway is self-regulating and able to scale to a perfect pattern in the dorsal half of bisected embryos. These findings illustrate how the complexity of an embryonic self-regulating system can be unraveled one gene at a time while keeping in mind the behavior of the whole system.
Bibliography
Ambrosio, A.L., Taelman, V.F., Lee, H.X., Metzinger, C.A., Coffinier, C. and De Robertis, E.M. (2008). Crossveinless-2 is a BMP feedback inhibitor that binds Chordin/BMP to regulate Xenopus embryonic patterning. Dev. Cell 15, 248-260.
Bachiller, D., Klingensmith, J., Kemp, C., Belo, J.A., Anderson, R.M., May, S.R., McMahon, J.A., McMahon, A.P., Harland, R., Rossant, J. and De Robertis, E.M. (2000). The organizer secreted factors Chordin and Noggin are required for forebrain development in the mouse. Nature 403, 658-661.
Bernard, C. (1865). Introduction to the Study of Experimental Biology, The McMillan Company. Translated into English in 1927.
Cho, K.W.Y, Blumberg, B., Steinbeisser, H. and De Robertis, E.M. (1991). Molecular Nature of Spemann's Organizer: the Role of the Xenopus Homeobox Gene goosecoid. Cell 67, 1111-1120.
Collavin, L., and Kirschner, M. W. (2003). The secreted Frizzled-related protein Sizzled functions as a negative feedback regulator of extreme ventral mesoderm. Development 130, 805-816.
Collins, F. (2006). The language of God: A Scientist Presents Evidence for Belief. Simon and Schuster, New York.
Conley, C.A., Silburn, R., Singer, M.A., Ralston, A., Rohwer-Nutter, D., Olson, D.J., Gelbart, W., Blair, S.S. (2000). Crossveinless 2 contains cysteine-rich domains and is required for high levels of BMP-like activity during the formation of the cross veins in Drosophila. Development 127, 3947-3959.
De Robertis, E.M. (2006). Spemann’s organizer and self-regulation in amphibian embryos. Nat. Rev. Mol. Cell Biol. 7, 296-302.
De Robertis, E.M. (2008). Evo-Devo: Variations on Ancestral themes. Cell 132, 185-195.
De Robertis, E.M. and Kuroda, H. (2004). Dorsal-ventral patterning and neural induction in Xenopus embryos. Annu. Rev. Cell Dev. Biol. Vol. 20, 285-308.
Dunham, I. et al. (2012) An integrated encyclopedia of DNA elements in the human genome. Nature 486, 57-74.
Ferguson, E.L. and Anderson, K.V. (1992). Localized enhancement and repression of the activity of the TGF-β family member, decapentaplegic, is necessary for dorsal-ventral pattern formation in the Drosophila embryo. Development 114, 583-597.
Gierer A. and Meinhardt H. (1972) A theory of biological pattern formation. Kybernetic 12, 30-39
Gould, S.J. (2002). The Structure of Evolutionary Theory. Harvard University Press, Cambridge, Massachusetts.
Gurdon, J.B. and Hopwood, N. (2000). The introduction of Xenopus laevis into developmental biology: of empire, pregnancy testing and ribosomal genes. Int. J. Dev. Biol. 441, 43-50.
Hemmati-Brivanlou, A., Kelly, O.G., and Melton, D.A. (1994). Follistatin, an antagonist of activin, is expressed in the Spemann Organizer and displays direct neuralizing activity. Cell 77, 283-295.
Holley, S.A., Jackson, P.D., Sasai, Y., Lu, B., De Robertis, E.M., Hoffman, F.M. and Ferguson, E.L. (1995). A conserved system for dorsal-ventral patterning in insects and vertebrates involving short gastrulation and chordin. Nature 376, 249-253.
Lee, H.X., Ambrosio, A.L., Reversade, B. and De Robertis, E.M. (2006). Embryonic dorsal-ventral signaling: secreted Frizzled-related proteins as inhibitors of Tolloid proteinases. Cell 124, 147-159.
Lee, H.X., Mendes, F.A., Plouhinec, J.L. and De Robertis, E.M. (2009). Enzymatic regulation of pattern: BMP4 binds CUB domains of Tolloids and inhibits proteinase activity. Genes Dev. 23, 2551-2562.
Levander, G. (1938). A study of bone regeneration. Surg. Gynecol. Obstet. 67, 705-704.
Little, S.C. and Mullins, M.C. (2006). Extracellular modulation of BMP activity in patterning the dorsoventral axis. Birth Defects Research 78, 224-242.
Massagué, J. (2000). How cells read TGF-β signals. Nat. Rev. Mol. Cell Biol. 1, 169-178.
Meinhardt, H. (2008). Models of biological pattern formation: from elementary steps to the organization of embryonic axes. Curr. Top. Dev. Biol. 81, 1-63.
Moos, Jr., M., Wang, S. and Krinks, M. (1995). Anti-dorsalizing morphogenetic protein is a novel TGF-beta homolog expressed in the Spemann organizer. Development 121, 4293-4301.
Nieuwkoop, P.D. and Florschütz, P.A. (1950). Quelques caractères spéciaux de la gastrulation et de la neurulation de l'oeuf de Xenopus laevis, Daud. et de quelques autres Anoures. Arch. de Biol. 61, 113-150.
Nüsslein-Volhard, C. and Wieschaus, E. (1980). Mutations affecting segment number and polarity in Drosophila. Nature 287, 795-801.
Oelgeschläger, M., Reversade, B., Larraín, J., Little, S., Mullins, M.C. and De Robertis, E.M. (2003). The pro-BMP activity of Twisted gastrulation is independent of BMP binding. Development 130, 4047-4056.
Piccolo, S., Sasai, Y., Lu, B. and De Robertis, E.M. (1996). Dorsoventral patterning in Xenopus: Inhibition of ventral signals by direct binding of Chordin to BMP-4. Cell 86, 589-598.
Piccolo, S., Agius, E., Lu, B., Goodman, S., Dale, L. and De Robertis, E.M. (1997). Cleavage of Chordin by the Xolloid metalloprotease suggests a role for proteolytic processing in the regulation of Spemann organizer activity. Cell 91, 407-416.
Ploper, D., Lee, H.X. and De Robertis, E.M. (2011). Dorsal-ventral patterning: Crescent is a dorsally secreted Frizzled-related protein that competitively inhibits Tolloid proteases. Dev. Biol. 352, 317-328.
Plouhinec, J.L. and De Robertis, E.M. (2009). Systems biology of the self-regulating morphogenetic gradient of the Xenopus gastrula. In: Reading and Interpreting Gradients during Development. J. Briscoe, P. Lawrence and J.P. Vincent, Eds., Cold Spring Harb. Perspect. Biol. 1:a001701.
Reversade, B. and De Robertis, E.M. (2005). Regulation of ADMP and BMP2/4/7 at opposite embryonic poles generates a self-regulating morphogen field. Cell 123, 1147-1160.
Sasai, Y., Lu, B., Steinbeisser, H., Geissert, D., Gont, L.K., and De Robertis, E.M. (1994). Xenopus chordin: a novel dorsalizing factor activated by organizer-specific homeobox genes. Cell 79, 779-790.
Sasai, Y., Lu, B., Steinbeisser, H. and De Robertis, E.M. (1995). Regulation of neural induction by the Chd and BMP-4 antagonistic patterning signals in Xenopus. Nature 376, 333-336.
Serpe, M., Umulis, D., Ralston, A., Chen, J., Olson, D.J., Avanesov, A., Othmer, H., O’Connor, M.B. and Blair, S.S. (2008). The BMP-binding protein Crossveinless 2 is a short-range, concentration-dependent, biphasic modulator of BMP signaling in Drosophila. Dev. Cell 14, 940-953.
Smith, W.C. and Harland, R.M. (1992). Expression cloning of noggin, a new dorsalizing factor localized to the Spemann organizer in Xenopus embryos. Cell 70, 829-840.
Spemann, H. (1928). Embryonic Development and Induction. Yale University Press, New Haven, Conn., reprinted by Hafner Publishing Company, New York, 1962.
Spemann, H., and Mangold, H. (1924). Induction of embryonic primordial by implantation of organizers from a different species. Roux’s Arch. Entw. Mech. 100, 599-638. Reprinted and translated in Int. J. Dev. Biol. Special Issue, E. M. De Robertis and J. Arechaga, Eds. (2001) 45, 13-31.
Urist, M. (1965). Bone: Formation by autoinduction. Science 150, 893-899.
Wozney, J.M., Rosen, V., Celeste, A.J., Mitsock, L.M., Whitters, M.J., Kriz, R.W., Hewick, R.M. and Wang, E.A. (1988). Novel regulators of bone formation: Molecular clones and activities. Science 242, 1528-1534.
Yabe, T., Shimizu, T., Muraoka, O., Bae, Y. K., Hirata, T., Nojima, H., Kawakami, A., Hirano, T., and Hibi, M. (2003). Ogon/Secreted Frizzled functions as a negative feedback regulator of Bmp signaling. Development 130, 2705-2716.
Xenopus chordin: a novel dorsalizing factor activated
by organizer-specific homeobox genes
Yoshiki Sasai, Bin Lu, Herbert Steinbeisser, Douglas Geissert, Linda K. Gont and E.M. De Robertis
Cell 79, 779-790 (1994)
A Xenopus gene whose expression can be activated by the organizer-specific
homeobox genes goosecoid and Xnot2 was isolated by differential
screening. The chordin gene encodes a novel protein of 941 amino acids that
has a signal sequence and four Cys-rich domains. The expression of chordin
starts in Spemann's organizer subsequent to that of goosecoid, and its
induction by activin requires de novo protein synthesis. Microinjection
of chordin mRNA induces twinned axes and can completely rescue axial
development in ventralized embryos. This molecule is a potent dorsalizing factor
that is expressed at the right time and in the right place to regulate cell-cell
interactions in the organizing centers of head, trunk, and tail development.
Expression pattern of chordin in the Xenopus
Regulation of neural induction by the Chd and Bmp-4 antagonistic patterning
signals in Xenopus
Yoshiki Sasai, Bin Lu, Herbert Steinbeisser and E.M. De Robertis
Nature 376, 333-336 (1995)
In Drosophila the amount of neurogenic ectoderm, from
which the central nervous system (CNS) derives, is regulated by a dorsal-ventral
system of positional information in which two secreted molecules of antagonistic
functions, decapentaplegic (dpp) and short-gastrulation (sog), play fundamental
roles. The vertebrate homologue of dpp is either bmp-4 or bmp-2,
and the homologue of sog is chd (s-chordin). In Xenopus
the CNS is induced by signals emanating from the organizer, and two proteins
secreted by the organizer, noggin and follistatin, have been shown to induce
neural tissue in animal-cap assays. Here we report that Chd, another organizer-specific
secreted factor, has neuralizing activity and that this activity can be antagonized
by Bmp-4. Inhibition of the function of the endogenous Bmp-4 present in the
animal cap also leads to neural differentiation. We suggest that conserved molecular
mechanisms involving chd/sog and bmp-4/dpp gene products pattern
the ecdoderm in Xenopus and in Drosophila.
Dorsoventral patterning in Xenopus: inhibition of ventral signals by direct
binding of chordin to BMP-4
Stefano Piccolo, Yoshiki Sasai, Bin Lu, and E.M. De Robertis
Cell 86, 589-598 (1996)
Chordin (Chd) is an abundant protein secreted by Spemann organizer
tissue during gastrulation. Chd antagonizes signaling by mature bone morphogenetic
proteins (BMPs) by blocking binding to their receptors. Recombinant Xenopus
Chd binds to BMP-4 with high affinity (KD 3 x
10-10 M), binding specifically to BMPs but not
to activin or TGF-b1. Chd protein is able to dorsalize
mesoderm and to neuralize ectoderm in Xenopus gastrula explants at 1 nM. We
propose that the noncell-autonomous effects of Spemann's organizer on dorsoventral
patterning are executed in part by diffusible signals that directly bind to
and neutralize ventral BMPs during gastrulation.
BMP-binding modules in chordin: a model for signalling regulation in the extracellular
space
Juan Larrain, Daniel Bachiller, Bin Lu, Eric Agius, Stefano Piccolo and E. M. De Robertis
Development 127, 821-830 (2000)
A number of genetic and molecular studies have implicated Chordin
in the regulation of dorsoventral patterning during gastrulation. Chordin, a
BMP antagonist of 120 kDa, contains four small (about 70 amino acids each) cysteine-rich
domains (CRs) of unknown function. In this study, we show that the Chordin CRs
define a novel protein module for the binding and regulation of BMPs. The biological
activity of Chordin resides in the CRs, especially in CR1 and CR3, which have
dorsalizing activity in Xenopus embryo assays and bind BMP4 with dissociation
constants in the nanomolar range. The activity of individual CRs, however, is
5- to 10-fold lower than that of full-length Chordin. These results shed light
on the molecular mechanism by which Chordin/BMP complexes are regulated by the
metalloprotease Xolloid, which cleaves in the vicinity of CR1 and CR3 and would
release CR/BMP complexes with lower anti-BMP activity than intact Chordin. CR
domains are found in other extracellular proteins such as procollagens. Full-length
Xenopus procollagen IIA mRNA has dorsalizing activity in embryo
microinjection assays and the CR domain is required for this activity. Similarly,
a C. elegans cDNA containing five CR domains induces secondary axes in
injected Xenopus embryos. These results suggest that CR modules may function
in a number of extracellular proteins to regulate growth factor signalling.
The organizer factors Chordin and Noggin are required for mouse forebrain
development.
Daniel Bachiller, John Klingensmith, Caroline Kemp, Jose A. Belo, R .M. Anderson, S.R. May, J. A. McMahon, A. P. McMahon, Richard Harland, Janet Rossant,
and E. M. De Robertis, E.M.
Nature 403, 658-661 (2000)
In mice, there is evidence suggesting that the development
of head and trunk structures is organized by distinctly separated cell populations.
The head organizer is located in the anterior visceral endoderm (AVE) and the
trunk organizer in the node and anterior primitive streak. In amphibians, Spemann's
organizer, which is homologous to the node, partially overlaps with anterior
endoderm cells expressing homologues of the AVE markers cerberus, Hex and Hesx1.
For mice, this raises the question of whether the AVE and node are independent
of each other, as suggested by their anatomical separation, or functionally
interdependent as is the case in amphibians. Chordin and Noggin are secreted
bone morphogenetic protein (BMP) antagonists expressed in the mouse node, but
not in the AVE. Here we show that mice double-homozygous mutants that are for
chordin and noggin display severe defects in the development of the prosencephalon.
The results show that BMP antagonists in the node and its derivatives are required
for head development.
The establishment of Spemann's organizer and patterning of
the vertebrate embryo
De Robertis, E.M., Larrain, J., Oelgeschlager, M. and
Wessely, O.
Nature Reviews Genetics 1, 171-181 (2000)
Molecular studies have begun to unravel the sequential cell-cell
signalling events that establish the dorsal-ventral, or 'back-to-belly', axis
of vertebrate animals. In Xenopus and zebrafish, these events start with the
movement of membrane vesicles associated with dorsal determinants. This mediates
the induction of mesoderm by generating gradients of growth factors. Dorsal
mesoderm then becomes a signalling centre, the Spemann's organizer, which secretes
several antagonists of growth-factor signalling. Recent studies have led to
new models for the regulation of cell-cell signalling during development, which
may also apply to the homeostasis of adult tissues.
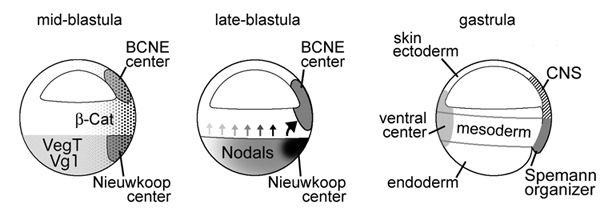
Neural induction in the absence of mesoderm: beta-catenin-dependent expression
of secreted BMP antagonists at the blastula stage in Xenopus
Oliver Wessely, Eric Agius, Michael Oelgeschlager, Edgar M. Pera and E. M. De Robertis
Developmental Biology 234, 161-173 (2001)
A growing body of work indicates that neural induction may
be initiated prior to the establishment of the gastrula mesodermal organizer.
Here, we examine neural induction in Xenopus embryos in which mesoderm induction
has been blocked by Cerberus-short, a reagent that specifically inhibits Nodal-related
(Xnr) signals. We find that extensive neural structures with cyclopic eyes and
brain tissue are formed despite the absence of mesoderm. This neural induction
correlates with the expression of chordin and other BMP inhibitors-such as noggin,
follistatin, and Xnr3-at the blastula stage, and requires beta-Catenin signaling.
Activation of the beta-Catenin pathway by mRNA microinjections or by treatment
with LiCl leads to differentiation of neurons, as well as neural crest, in ectodermal
explants. Xnr signals are required for the maintenance, but not for the initiation,
of BMP antagonist expression. Recent work has demonstrated a role for beta-Catenin
signaling in neural induction mediated by the transcriptional down-regulation
of BMP-4 expression. The present results suggest an additional function for
beta-Catenin, the early activation of expression of secreted BMP antagonists,
such as Chordin, in a preorganizer region in the dorsal side of the Xenopus
blastula.
Proteolytic cleavage of Chordin as a switch for the dual activities of Twisted
gastrulation in BMP signaling
Juan Larrain, Michael Oelgeschlager, Nan L. Ketpura, N.I., Bruno Reversade,
Lise Zakin, and E. M. De Robertis
Development 128, 4439-4447 (2001)
Dorsoventral patterning is regulated by a system of interacting
secreted proteins involving BMP, Chordin, Xolloid and Twisted gastrulation (Tsg).
We have analyzed the molecular mechanism by which Tsg regulates BMP signaling.
Overexpression of Tsg mRNA in Xenopus embryos has ventralizing effects similar
to Xolloid, a metalloprotease that cleaves Chordin. In embryos dorsalized by
LiCl treatment, microinjection of Xolloid or Tsg mRNA restores the formation
of trunk-tail structures, indicating an increase in BMP signaling. Microinjection
of Tsg mRNA leads to the degradation of endogenous Chordin fragments generated
by Xolloid. The ventralizing activities of Tsg require an endogenous Xolloid-like
activity, as they can be blocked by a dominant-negative Xolloid mutant. A BMP-receptor
binding assay revealed that Tsg has two distinct and sequential activities on
BMP signaling. First, Tsg makes Chordin a better BMP antagonist by forming a
ternary complex that prevents binding of BMP to its cognate receptor. Second,
after cleavage of Chordin by Xolloid, Tsg competes the residual anti-BMP activity
of Chordin fragments and facilitates their degradation. This molecular pathway,
in which Xolloid switches the activity of Tsg from a BMP antagonist to a pro-BMP
signal once all endogenous full-length Chordin is degraded, may help explain
how sharp borders between embryonic territories are generated.
Cerberus-like is a secreted BMP and nodal antagonist not essential for mouse development
José António Belo, Daniel Bachiller, Eric Agius, Caroline Kemp, A.C. Borges, S. Marques, S. Piccolo S and E.M. De Robertis
Genesis 26, 265-270 (2000)
Mouse cerberus-like (cer-l) is a member of the Cerberus/Dan family of secreted factors. As other members of this family of proteins, Cer-l functions in the extracellular space, inhibiting signaling molecules. Here we show that the neural-inducing and mesoderm-inhibiting activities of Cer-l result from specific binding to BMP and Nodal molecules, respectively. These properties resemble the ones from the related factor Xenopus Cerberus. However, Xenopus Cerberus in addition to BMP4 and Nodal also binds to and inhibits Wnt proteins. We show that Cer-l does not directly inhibit Wnt signals. A null allele of the mouse Cer-l gene was generated by targeted inactivation in ES cells. Homozygous embryos show no anterior patterning defects, are born alive, and are fertile. Since mouse Cer-l and Xenopus Cerberus differ in biochemical activities, we propose the existence of additional members of this family of inhibitors, which may compensate for the loss of cer-l..
The role of chordin/Bmp signals in mammalian pharyngeal development and DiGeorge
syndrome
Daniel Bachiller, John Klingensmith, N. Shneyder, Uyen Tran,
R. Anderson, Janet Rossant, and E. M. De Robertis
Development 130, 3567-3578 (2003)
The chordin/Bmp system provides one of the best examples of
extracellular signaling regulation in animal development. We present the phenotype
produced by the targeted inactivation of the chordin gene in mouse. Chordin
homozygous mutant mice show, at low penetrance, early lethality and a ventralized
gastrulation phenotype. The mutant embryos that survive die perinatally, displaying
an extensive array of malformations that encompass most features of DiGeorge
and Velo-Cardio-Facial syndromes in humans. Chordin secreted by the mesendoderm
is required for the correct expression of Tbx1 and other transcription factors
involved in the development of the pharyngeal region. The chordin mutation provides
a mouse model for head and neck congenital malformations that frequently occur
in humans and suggests that chordin/Bmp signaling may participate in their pathogenesis.
Integrin-a3 mediates binding of Chordin to the cell surface and promotes its entocytosis
Juan Larrain, Carlos Brown and E. M. De Robertis
EMBO Reports 4, 813-818 (2003)
Dorsoventral patterning in animal development is regulated by a morphogenetic gradient of Bone morphogenetic protein signalling, which is established by a set of proteins that are conserved from Drosophila to vertebrates. These include Chordin (Chd)/Short gastrulation, Xolloid/Tolloid and Twisted gastrulation. Here, we report the identification of a cell-surface component of this morphogenetic pathway. Prompted by the observation that Chd protein bound to the surface of certain cell lines with subnanomolar affinity, we identified two cell-surface proteins that bind to Chd, one of which corresponds to Integrin-alpha3. Integrin-alpha3 and Chd are co-expressed in the Xenopus embryo. Transfection of Integrin-alpha3 increased the binding of Chd to the cell surface, which was competed by an excess of soluble Integrin-alpha3. After binding to the cell surface, Chd was translocated into intracellular endocytic compartments in a temperature-dependent manner. We propose that Integrin-alpha3 may regulate the concentration of Chd protein in the extracellular space by endocytosis.
Chordin is required for the Spemann organizer transplantation phenomenon in
Xenopus embryos
Michael Oelgeschlager, Hiroki Kuroda, Bruno Reversade and E. M. De Robertis
Developmental Cell 4, 219-230 (2003)
We analyzed the Chordin requirement in Xenopus development.
Targeting of both chordin Xenopus laevis pseudoalleles with morpholino antisense
oligomers (Chd-MO) markedly decreased Chordin production. Embryos developed
with moderately reduced dorsoanterior structures and expanded ventroposterior
tissues, phenocopying the zebrafish chordino mutant. A strong requirement for
Chordin in dorsal development was revealed by experimental manipulations. First,
dorsalization by lithium chloride treatment was completely blocked by Chd-MO.
Second, Chd-MO inhibited elongation and muscle differentiation in Activin-treated
animal caps. Third, Chd-MO completely blocked the induction of the central nervous
system (CNS), somites, and notochord by organizer tissue transplanted to the
ventral side of host embryos. Unexpectedly, transplantations into the dorsal
side revealed a cell-autonomous requirement of Chordin for neural plate differentiation.
Analysis of Spemann organizer formation in Xenopus embryos by cDNA macroarrays
Oliver Wessely, James I. Kim, Douglas Geissert, Uyen Tran and E.M. De
Robertis
Developmental Biology 269, 552-566 (2004)
The understanding of vertebrate development has greatly benefited
from the study of gastrulation in the Xenopus embryo. Over the years, the molecular
dissection of the Spemann organizer has proven to be a very fruitful source
for gene discovery. Here, we report a comprehensive screen of gene expression
in the Xenopus gastrula using cDNA macroarrays. Nylon filters containing more
than 72000 cDNAs from a gastrula stage library were hybridized with differential
probes from embryos in which organizer induction had been inhibited by reducing
Nodal-related or maternal beta-Catenin signaling. Combining the changes in gene
expression levels caused by these two major signaling pathways in a single graph
identified both known and novel dorsoventral regulated genes. The most highly
enriched organizer-specific genes were the secreted molecules chordin and Xnr-3,
followed by the transmembrane protein paraxial protocadherin (PAPC). Ventral-specific
abundant cDNAs included S10-40-H5, members of the Hyaluronan synthase family,
Xvent-2 and XFD2/FoxI1. A differential probe of dorsal and ventral lips identified
many more organizer-specific cDNAs than the screens inhibiting Nodal-related
and beta-Catenin signaling, suggesting that additional, as yet uncharacterized
signaling pathways, contribute to organizer formation. Finally, extension of
this approach to the blastula preorganizer signaling center identified the transcription
factor pintallavis/FoxA2 as a new preorganizer component.
Neural Induction in Xenopus: Requirement for Ectodermal and Endomesodermal
Signals via Chordin, Noggin, ß-Catenin, and Cerberus
Hiroki Kuroda, Oliver Wessely and E. M. De Robertis
PLoS Biology 2, 623-634 (2004)
The origin of the signals that induce the differentiation of the central nervous
system (CNS) is a long-standing question in vertebrate embryology. Here we show
that Xenopus neural induction starts earlier than previously thought, at the
blastula stage, and requires the combined activity of two distinct signaling
centers. One is the well-known Nieuwkoop center, located in dorsal-vegetal cells,
which expresses Nodal-related endomesodermal inducers. The other is a blastula
Chordin- and Noggin-expressing (BCNE) center located in dorsal animal cells
that contains both prospective neuroectoderm and Spemann organizer precursor
cells. Both centers are downstream of the early ß-Catenin signal. Molecular
analyses demonstrated that the BCNE center was distinct from the Nieuwkoop center,
and that the Nieuwkoop center expressed the secreted protein Cerberus (Cer).
We found that explanted blastula dorsal animal cap cells that have not yet contacted
a mesodermal substratum can, when cultured in saline solution, express definitive
neural markers and differentiate histologically into CNS tissue. Transplantation
experiments showed that the BCNE region was required for brain formation, even
though it lacked CNS-inducing activity when transplanted ventrally. Cell-lineage
studies demonstrated that BCNE cells give rise to a large part of the brain
and retina and, in more posterior regions of the embryo, to floor plate and
notochord. Loss-of-function experiments with antisense morpholino oligos (MO)
showed that the CNS that forms in mesoderm-less Xenopus embryos (generated by
injection with Cerberus-Short [CerS] mRNA) required Chordin (Chd), Noggin (Nog),
and their upstream regulator ß-Catenin. When mesoderm involution was prevented
in dorsal marginal-zone explants, the anterior neural tissue formed in ectoderm
was derived from BCNE cells and had a complete requirement for Chd. By injecting
Chd morpholino oligos (Chd-MO) into prospective neuroectoderm and Cerberus morpholino
oligos (Cer-MO) into prospective endomesoderm at the 8-cell stage, we showed
that both layers cooperate in CNS formation. The results suggest a model for
neural induction in Xenopus in which an early blastula ß-Catenin signal
predisposes the prospective neuroectoderm to neural induction by endomesodermal
signals emanating from Spemann's organizer.
Dorsal-ventral patterning and neural induction in Xenopus embryos
E. M. De Robertis and Hiroki Kuroda
Annu. Rev. Cell Dev. Biol. 20, 285-308 (2004)
We review the current status of research in dorsal-ventral
(D-V) patterning in vertebrates. Emphasis is placed on recent work on Xenopus,
which provides a paradigm for vertebrate development based on a rich heritage
of experimental embryology. D-V patterning starts much earlier than previously
thought, under the influence of a dorsal nuclear -Catenin signal. At mid-blastula
two signaling centers are present on the dorsal side: The prospective neuroectoderm
expresses bone morphogenetic protein (BMP) antagonists, and the future dorsal
endoderm secretes Nodal-related mesoderm-inducing factors. When dorsal mesoderm
is formed at gastrula, a cocktail of growth factor antagonists is secreted by
the Spemann organizer and further patterns the embryo. A ventral gastrula signaling
center opposes the actions of the dorsal organizer, and another set of secreted
antagonists is produced ventrally under the control of BMP4. The early dorsal
-Catenin signal inhibits BMP expression at the transcriptional level and promotes
expression of secreted BMP antagonists in the prospective central nervous system
(CNS). In the absence of mesoderm, expression of Chordin and Noggin in ectoderm
is required for anterior CNS formation. FGF (fibroblast growth factor) and IGF
(insulin-like growth factor) signals are also potent neural inducers. Neural
induction by anti-BMPs such as Chordin requires mitogen-activated protein kinase
(MAPK) activation mediated by FGF and IGF. These multiple signals can be integrated
at the level of Smad1. Phosphorylation by BMP receptor stimulates Smad1 transcriptional
activity, whereas phosphorylation by MAPK has the opposite effect. Neural tissue
is formed only at very low levels of activity of BMP-transducing Smads, which
require the combination of both low BMP levels and high MAPK signals. Many of
the molecular players that regulate D-V patterning via regulation of BMP signaling
have been conserved between Drosophila and the vertebrates.
|
|
Figure 1. The Spemann-Mangold organizer
experiment repeated in Xenopus laevis. |
|
Figure 2. Organizer formation in Xenopus
laevis. |
|
|
Figure 3. Proteins secreted by dorsal
(Spemann organizer) or ventral gastrula signaling centers. |
|
|
Figure 4. Cysteine-rich (CR) BMP-binding
modules in Chordin and Crossveinless-2. |
|
|
Figure 5. Integration of multiple signaling
pathways at the level of Smad1 phosphorylation during neural induction. |
|
|
Figure 6. Signaling centers at blastula
and gastrula that have critical roles for body plan formation in Xenopus. |
|
|
Figure 7. Inversion in the course of evolution
of a conserved network of extracellular regulators of BMP/Dpp signaling
involved in D-V patterning. |
Expression of Siamois and Twin in the blastula Chordin/Noggin signaling center is required for brain formation in Xenopus laevis embryos.
H. Ishibashi, N. Matsumura, H. Hanafusa, K. Matsumoto, E. M. De Robertis and Hiroki Kuroda
Mech. Dev. 125, 58-66 (2008)
The blastula Chordin- and Noggin-expressing (BCNE) center located in the dorsal animal region of the Xenopus blastula embryo contains both prospective anterior neuroectoderm and Spemann organizer precursor cells. Here we show that, contrary to previous reports, the canonical Wnt target homeobox genes, Double knockdown of these genes using antisense morpholinos in Xenopus laevis blocked head formation, reduced the expression of the other BCNE center genes, upregulated Bmp4 expression, and nullified hyperdorsalization by lithium chloride. Moreover, gain- and loss-of-function experiments showed that Siamois and Twin expression is repressed by the vegetal transcription factor VegT. We propose that VegT expression causes maternal beta-Catenin signals to restrict Siamois and Twin expression to the BCNE region. A two-step inhibition of BMP signals by Siamois and Twin-- first by transcriptional repression of Bmp4 and then by activation of the expression of the BMP inhibitors Chordin and Noggin--in the BCNE center is required for head formation.
Spemann's organizer and the self-regulation of embryonic fields
E. M. De Robertis
Mech. Dev. 126, 925-941 (2009)
Embryos and developing organs have the remarkable ability of self-regenerating after experimental manipulations. In the Xenopus blastula half-embryos can regenerate the missing part, producing identical twins. Studies on the molecular nature of Spemann’s organizer have revealed that self-regulation results from the battle between two signaling centers under reciprocal transcriptional control. Long-range communication between the dorsal and ventral sides is mediated by the action of growth factor antagonists – such as the BMP antagonist Chordin – that regulate the flow of BMPs within the embryonic morphogenetic field. BMPs secreted by the dorsal Spemann organizer tissue are released by metalloproteinases of the Tolloid family, which cleave Chordin at a distance of where they were produced. The dorsal center secretes Chordin, Noggin, BMP2 and ADMP. The ventral center of the embryo secretes BMP4, BMP7, Sizzled, Crossveinless-2 and Tolloid-related. Crossveinless-2 binds Chordin/BMP complexes, facilitating their flow towards the ventral side, where BMPs are released by Tolloid allowing peak BMP signaling. Self-regulation occurs because transcription of ventral genes is induced by BMP while transcription of dorsal genes is repressed by BMP signals. This assures that for each action of Spemann’s organizer there is a reaction in the ventral side of the embryo. Because both dorsal and ventral centers express proteins of similar biochemical activities, they can compensate for each other. A novel biochemical pathway of extracellular growth factor signaling regulation has emerged from these studies in Xenopus. This remarkable dorsal-ventral positional information network has been conserved in evolution and is ancestral to all bilateral animals.


Last updated: March 2019



















![[Chordin st10_75]](chd10_75s.jpg)
![[Chordin st11_5]](chd11_5s.jpg)
![[Chordin st13]](chd13s.jpg)
![[Chordin st26]](chd26s.jpg)
![[Chordin st33]](chd33s.jpg)